Abstract
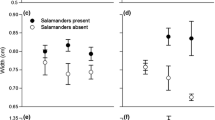
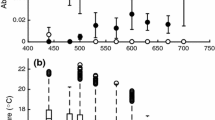
Many of the far-reaching impacts of climate change on ecosystem function will be due to alterations in species interactions. However, our understanding of the effects of temperature on the dynamics of interactions between species is largely inadequate. Inducible defences persist in prey populations because defensive traits increase survival in the presence of predators but are costly when they are absent. Large-scale changes in the thermal climate are likely to alter the costs or benefits of these defences for ectotherms, whose physiological processes are driven by environmental temperature. A shift in costs of defensive traits would affect not only predator–prey interactions, but also the strength of selection for inducible defences in natural populations. We investigate the effect of temperature on the costs of behavioural defences in larvae of the marine toad, Rhinella marinus. Larvae were reared in the presence or absence of predator cues at both 25 and 30 °C. When exposed to predation cues, larvae reduced activity and spent less time feeding. Exposure to predation cues also reduced metabolic rate, presumably as a by-product of reducing activity levels. Larvae exposed to predation cues also grew more slowly, were smaller at metamorphosis and were poorer jumpers after metamorphosis—three traits associated with fitness in post-metamorphic anurans. We found that the costs of behavioural defences, in terms of larval growth, post-metamorphic size and jumping performance, were exacerbated at cooler temperatures. The thermal sensitivity of costs associated with defensive traits may explain geographic variation in plasticity of defensive traits in other species and suggests that changes in environmental temperature associated with climate change may affect predator–prey interactions in subtle ways not previously considered.




Similar content being viewed by others
References
Alexander RM (1982) Locomotion of animals. Blackie, Glasgow
Alton LA, White CR, Wilson RS, Franklin CE (2011) The energetic cost of exposure to UV radiation for tadpoles is greater when they live with predators. Funct Ecol 26:94–103
Beketov MA, Liess M (2007) Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipens. Ecol Entomol 32:405–410
Callahan HS, Maughan H, Steiner UK (2008) Phenotypic plasticity, costs of phenotypes, and costs of plasticity toward an integrative view. Year Evolut Biol 1133:44–66
Capellan E, Nicieza AG (2007) Trade-offs across life stages: does predator-induced hatching plasticity reduce anuran post-metamorphic performance? Evol Ecol 21:445–458
Chelgren ND, Rosenberg DK, Heppel SS, Gitelman AI (2006) Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol Appl 16:250–261
Child T, Phillips BL, Shine R (2009) Does desiccation risk drive the distribution of juvenile cane toads (Bufo marinus) in tropical Australia? J Trop Ecol 25:193–200
Crawley MJ (2007) The R book. Wiley, West Sussex
Dewitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465–480
Dewitt TJ, Sheiner SM (2004) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, Oxford
Ficetola GF, De Bernardi F (2006) Trade-off between larval development rate and post metamorphic traits in the frog Rana latastei. Evol Ecol 20:143–158
Fraker ME (2008) The effect of hunger on the strength and duration of the antipredator behavioural response of green frog (Rana clamitans) tadpoles. Behav Ecol Sociobiol 62:1202–1205
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Graham RW, Grimm EC (1990) Effects of global climate change on the patterns of terrestrial biological communities. Trends Ecol Evol 5:289–292
Hagman M, Shine R (2008) Understanding the toad code: behavioural responses of cane toad (Chaunus marinus) larvae and metamorphs to chemical cues. Austral Ecol 33:37–44
Hagman M, Shine R (2009a) Factors influencing responses to alarm pheromone by larvae of invasive cane toads, Bufo marinus. J Chem Ecol 35:265–271
Hagman M, Shine R (2009b) Larval alarm pheromones as a potential control for invasive cane toads (Bufo marinus) in tropical Australia. Chemoecology 19:211–217
Hagman M, Hayes RA, Capon RJ, Shine R (2009) Alarm cues experienced by cane toad tadpoles affect post-metamorphic morphology and chemical defences. Funct Ecol 23:126–132
Hammill E, Rogers A, Beckerman AP (2008) Costs, benefits and the evolution of inducible defences: a case study with Daphnia pulex. J Evol Biol 21:705–715
Havel JE, Dodson SI (1984) Chaoborus predation on typical and spined morphs of Daphnia pulex: behavioural observations. Limnol Oceanogr 29:487–494
Hughes L (2000) Biological consequences of global warming: is the signal already apparent. Trends Ecol Evol 15:56–61
Juliano SA, Reminger L (1992) The relationship between vulnerability to predation and behavior of larval treehole mosquitos —geographic and ontogenic differences. Oikos 63:465–476
Köster M, Krause C, Parrenhöfer GA (2008) Time-series measurements of oxygen consumption of copepod nauplii. Mar Ecol Prog Ser 353:157–164
Laurila A, Pakkasmaa S, Merila J (2006) Population divergence in growth rate and antipredator defences in Rana arvalis. Oecologia 147:585–595
Laurila A, Lindgren B, Laugen AT (2008) Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89:1399–1413
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions—what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Mccarty JP (2001) Ecological conseqences of recent climate change. Conserv Biol 15:320–331
McCollum SA, Van Buskirk J (1996) Costs and benefits of a predator-induced polyphenism in the Grey Treefrog Hyla Chrysoscelis. Evolution 50:583–593
Mclaughlin JF, Hellmann JJ, Boggs CL, Ehrlich PR (2002) Climate change hastens population extinctions. Proc Natl Acad Sci USA 99:6070–6074
Mcpeek MA (2004) The growth/predation risk trade-off: so what is the mechanism? Am Nat 163:E88–E111
Mcpeek MA, Grace M, Richardson JML (2001) Physiological and behavioural responses to predators shape the growth/predation risk trade-off in damselflies. Ecology 82:1535–1545
Niehaus AC, Wilson RS, Seebacher F, Franklin CE (2011) Striped marsh frog (Limnodynastes peronii) tadpoles do not acclimate metabolic performance to thermal variability. J Exp Biol 214:1965–1970
Nilsson PA, Bronmark C, Pettersson LB (1995) Benefits of a predator-induced morphology in crucian carp. Oecologia 104:291–296
R Development Core Team (2008) R: a language and environment for statistical computing. R: foundation for statistical computing, Vienna, Austria
Riley JP, Chester R (1971) Introduction fo marine chemistry. Academic Pres, London
Sih A (1992) Prey uncertainty and the balancing of antipredator and feeding needs. Am Nat 139:1052–1069
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval american toads to an odonate predator. Ecology 71:2313–2322
Steiner UK (2007) Investment in defense and cost of predator-induced defense along a resource gradient. Oecologia 152:201–210
Steiner UK, Van Buskirk J (2009) Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLoS One 4:e6160
Stoks R, De Block M, Mcpeek MA (2005) Alternative growth and energy storage responses to mortality threats in damselflies. Ecol Lett 8:1307–1316
Turner AM (1996) Freshwater snails alter habitat use in response to predation. Anim Behav 51:747–756
Turner AM (1997) Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behav Ecol 8:120–125
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573. doi:10.1126/science.aaa4984
Van Buskirk J (2000) The costs of an inducible defense in anuran larvae. Ecology 81:2813–2821
van Uitregt VO, Hurst T, Wilson RS (2011) Reduced size and starvation resistance in adult mosquitoes, Aedes notoscriptus exposed to predation cues as larvae. J Anim Ecol. doi:10.1111/j.1365-2656.2011.01880.x
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality-rates mediated by foraging activity. American Naturalist 142:242–272
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct Ecol 19:238–244
Acknowledgements
This study was supported by the ARC Discovery Project DP120101215.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
van Uitregt, V.O., Alton, L.A., Heiniger, J. et al. Warmer temperatures reduce the costs of inducible defences in the marine toad, Rhinella marinus . J Comp Physiol B 186, 123–130 (2016). https://doi.org/10.1007/s00360-015-0938-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0938-0