Abstract
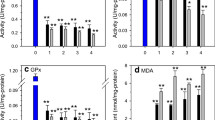
To assess the toxicity of heavy metal pollution to marine intertidal shellfish, enzymatic responses and lipid peroxidation were investigated in the clam Mactra vereformis exposed to cadmium under laboratory conditions. Three antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT; glutathione peroxidase, GPx), two immune defense enzymes (acid phosphatase, ACP; alkaline phosphatase, ALP), and one lipid peroxidation product (malondialdehyde, MDA) were measured in the gills and the hepatopancreas of the clam exposed to 0, 25, 75, and 125 μg/L cadmium for 0, 1, 3, 5, and 7 d. The results show that the concentrations of antioxidant enzymes in the organs soared to a peak value on the first day and then decreased afterwards in most cases. CAT and GPx activities in the hepatopancreas were higher than in the gills, but the SOD activity was lower in the hepatopancreas. ACP activity was unchanged until Day 3 in the hepatopancreas and until Day 5 in gills, when it began to increase. ALP activity showed no significant relationship with Cd treatment. MDA concentrations increased in the two tissues after Cd exposure, peaked on Day 3 in gills, and on Day 5 in hepatopancreas. These observations show that changes in the activities of antioxidant enzymes and ACP reflect the time course of oxidative stress in the clam caused by Cd, and could be used as potential biomarkers for ecotoxicological bioassays of heavy metals.
Similar content being viewed by others
References
Almeida J A, Diniz Y S, Marques S F G, Faine L A, Ribas B O, Burneiko R C, Novelli E L B. 2002. The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ. Int., 27: 673–679.
Bebianno M J, Company R, Serafim A, Cosson R P, Fiala-Medoni A. 2005. Antioxidant systems and lipid peroxidation in Bathymodiolusazoricus from Mid-Atlantic Ridge hydrothermal vent fields. Aquat. Toxicol., 75: 354–373.
Bem E M, Mailer K, Elson C M. 1985. Influence of mercury (II), cadmium (II), methylmercury, and phenylmercury on the kinetic properties of rat liver glutathione peroxidase. Can. J. Biochem. Cell Biol., 63: 1 212–1 216.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248–254.
Brouwer M, Brouwer T H. 1998. Biochemical defense mechanisms against copper-induced oxidative damage in the blue crab, Callinectes sapidus. Arch. Biochem. Biophys., 351: 257–264.
Chen H B. 2005. Research and demonstration of the technologies of restoring the typical coastal zone tidal-flat habitat and the living resources in the Bohai Sea. Mar. Inform., 3: 20–23. (in Chinese with English abstract)
Chen J L, Liu W X, Liu S Z, Lin X M, Tao S. 2004. An evaluation on heavy metal contamination in the surface sediments in Bohai Sea. Mar. Sci., 28: 16–21. (in Chinese with English abstract)
Company R, Serafim A, Cosson R P, Camus L, Shillito B, Fiala-Médioni A, Bebianno M J. 2006. The effect of cadmium on antioxidant responses and the susceptibility to oxidative stress in the hydrothermal vent mussel Bathymodiolus azoricus. Mar. Biol., 148: 817–825.
Cossu C, Doyotte A, Jacquin M C, Babut M, Exinger A, Vasseur P. 1997. Glutathione reductase, seleniumdependent glutathione peroxidase, glutathione levels, and lipid peroxidation in freshwater bivalves, Unio tumidus, as biomarkers of aquatic contamination in field studies. Ecotoxi. Environ. Safe., 38: 122–131.
Fridovich I. 1998. Oxygen toxicity: a radical explanation. J. Exp. Biol., 210: 1 203–1 209.
Géret F, Serafim A, Barreira L, Bebianno M J. 2002a. Response of antioxidant systems to copper in the gills of the clam Ruditapes decussates. Mar. Environ. Res., 54: 413–417.
Géret F, Serafim A, Barreira L, Bebianno M J. 2002b. Effect of cadmium on antioxidant enzyme activities and lipid peroxidation in the gills of the clam Ruditapes decussatus. Biomarkers, 7: 242–256.
Géret F, Jouan A, Turpin V, Bebianno M J, Cosson R. 2002c. Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: the oyster (Crassostrea gigas) and the mussel (Mytilus edulis). Aquat. Living Resour., 15: 61–66.
Géret F, Serafim A, Bebianno M J. 2003. Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicol., 12: 417–426.
Gonzalez F, Ferez-Vidal M E, Arias J M, Mantoya E. 1994. Partial purification and biochemical properties of acid and alkaline phosphatases from Myxococcus coralloides. D. J. Appl. Bacteriol., 77: 567–573.
Góth L. 1991. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta, 196: 143–151.
Giguère A, Couillard Y, Campbell P G C, Perceval O, Hare L, Pinel-Alloul B, Pellerin J. 2003. Steady-state distribution of metals among metallothionein and other cytosolic ligands and links to cytotoxicity in bivalves living along a polymetallic gradient. Aquat. Toxicol., 64: 185–200.
Jing G, Li Y, Xie L P, Zhang R Q. 2006. Metal accumulation and enzyme activities in gills and digestive gland of pearl oyster (Pinctada fucata) exposed to copper. Comp. Biochem. Phys. C, 144: 184–190.
Kono Y, Fridovich I. 1982. Superoxide radical inhibits catalase. J. Biol. Chem., 257: 5 751–5 754.
Lesser M P. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu. Rev. Physiol., 68: 253–278.
Li S M, Hu D Y, Tang C S. 1996. Biological effect of metallothionein. Foreign Med. Sci. (Sec. Pathophysiol. Clin. Med.), 16: 36–38.
McCord J M, Fridovich I. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem., 244: 6 049–6 055.
Mazorra M T, Rubio J A, Blasco J. 2002. Acid and alkaline phosphatase activities in the clam Scrobicularia plana: kinetic characteristics and effects of heavy metals. Comp. Biochem. Phys. B, 131: 241–249.
Metian M, Hédouin L, Barbot C, Teyssié J L, Fowler S W, Goudard F, Bustamante P, Durand J P, Piéri J, Warnau M. 2005. Use of radiotracer techniques to study subcellular distribution of metals and radionuclides in bivalves from the Noumea Lagoon, New Caledonia. B. Environ. Contam. Tox., 75: 89–93.
Meng W, Qin Y W, Zheng B H, Zhang L. 2008. Heavy metal pollution in Tianjin Bohai Bay, China. J. Environ. Sci. China, 20: 814–819.
Nair V, Cooper C S, Vietti D E, Turner G A. 1986. The chemistry of lipid peroxidation metabolites: crosslinking reactions of malondialdehyde. Lipids, 21: 6–10.
Novelli E L B, Vieira E P, Rodrigues N L, Ribas B O. 1998. Risk assessment of cadmium toxicity on hepatic and renal tissues of rats. Environ. Res., 79: 102–105.
Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem., 95: 351–358.
Pan L Q, Zhang H X. 2006. Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comp. Biochem. Phys. C, 144: 67–75.
Pipe R K, Coles J A, Carissan F M M, Ramanathan K. 1999. Copper induced immunomodulation in the marine mussel, Mytilus edulis. Aquat. Toxicol., 46: 43–54.
Rajalakshmi S, Mohandas A. 2005. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicol. Environ. Saf., 62: 140–143.
Rajalakshmi S, Mohandas A. 2008. Impact of mercury on the activity pattern of a marker enzyme in a freshwater bivalve. The Environmentalist, 28: 249–252.
Regoli F, Orlando E. 1994. Accumulation and subcellular distribution of metals (Cu, Fe, Mn, Pb and Zn) in the Mediterranean mussel Mylilus galloprovincialis during a field transplant experiment. Mar. Pollut. Bull., 28: 592–600.
Regoli F, Principato G. 1995. Glutathione, glutathionedependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat. Toxicol., 31: 143–164.
Regoli F, Nigro M, Orlando E. 1998. Lysosomal and antioxidant responses to metals in the Antarctic scallop Adamussium colbecki. Aquat. Toxicol., 40: 375–392.
Rittschof D, McClellan-Green P. 2005. Molluscs as multidisciplinary models in environment toxicology. Mar. Pollut. Bull., 50: 369–373.
Shi D L, Wang W X. 2004. Understanding the differences in Cd and Zn bioaccumulation and subcellular storage among different populations of marine clams. Environ. Sci. Technol., 38: 449–456.
Silva A M M, Novelli E L B, Fascineli M L, Almeida J A. 1999. Impact of an environmentally realistic intake of water contaminants and superoxide formation on tissues of rats. Environ. Pollut., 105: 243–249.
Sinnhuber R O, Yu T C. 1958. Characterization of the red pigment formed in the 2-thiobarbituric acid determination of oxidative rancidity. Food Res., 23: 626–634.
Siraj Basha P, Usha Rani A. 2003. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotox. Environ. Safe., 56: 218–221.
Soldatov A A, Gostyukhina O L, Golovina I V. 2007. Antioxidant enzyme complex of tissues of the bivalve Mytilus galloprovincialis Lam. under normal and oxidative-stress conditions: A review. Appl. Biochem. Microbio., 43: 556–562.
Splittgerber A G, Tapple A L. 1979. Inhibition of glutathione peroxidase by cadmium and other metal ions. Arch. Biochem. Biophys., 197: 534–542.
Stohs S J, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radical Bio. Med., 18: 321–326.
Viarengo A. 1990. Heavy metal effects on lipid peroxidation in the tissues of Mytilus galloprovincialis Lam. Comp. Biochem. Phys. C, 97: 37–42.
Viarengo A, Nott J A. 1993. Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comp. Biochem. Phys. C, 104: 355–372.
Wang Y W, Liang L N, Shi J B, Jiang G B. 2005. Study on the contamination of heavy metals and their correlations in mollusks collected from coastal sites along the Chinese Bohai Sea. Environ. Int., 31: 1 103–1 113.
Wang C Y, Wang X L. 2007. Spatial distribution of dissolved Pb, Hg, Cd, Cu and As in the Bohai Sea. J. Environ. Sci-China, 19: 1 061–1 066.
Winston G W, Di Giulio R T. 1991. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol., 19: 137–161.
Wu F, Zhang G, Dominy P. 2003. Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot., 50: 67–78.
Xu H Z, Zhou C G, Ma Y A, Shang L S, Yao Z W, Li H. 2000. Environmental quality of deposits in offshore zone of China. Environ. Protect. Transportation, 21: 16–18. (in Chinese with English abstract)
Xu X D, Lin Z H, Li S Q. 2005. The studied of the heavy metal pollution of Jiaozhou Bay. Mar. Sci. 29: 48–53. (in Chinese with English abstract)
Yan X W, Zhang Y H, Zuo J P, Huo Z M, Yang F, Zhang G F, Yang D G, Guo H J. 2008. Artificial breeding technique of clam Mactra veneriformis in Northern coast in China. J. Dalian Fish. Univ., 23: 348–352. (in Chinese with English abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Basic Research Program of China (973 Program) (No. 2007CB407305), the Tianjin Program for Marine Development by Reliance on Science and Technology (No. kx2010-4), the National Marine Public Welfare Research Project of China (No. 200805069), the Natural Science Fundation for Creative Research Groups (No. 40821004), and the Knowledge Innovation Key Projects of Chinese Academy of Sciences (No. KZCX2-YW-Q07-03)
Rights and permissions
About this article
Cite this article
Wang, X., Yang, H., Liu, G. et al. Enzyme responses and lipid peroxidation in gills and hepatopancreas of clam Mactra vereformis, following cadmium exposure. Chin. J. Ocean. Limnol. 29, 981–989 (2011). https://doi.org/10.1007/s00343-011-0088-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-011-0088-5