Abstract
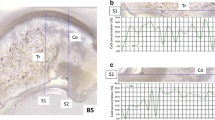
Synchrotron micro-X-ray Fluorescence has been used to map the metal distribution in selected bone fragments representative of remains associated with the Franklin expedition. In addition, laser ablation mass spectroscopy using a 25 μm diameter circular spot was employed to compare the Pb isotope distributions in small regions within the bone fragments. The X-ray Fluorescence mapping shows Pb to be widely distributed in the bone while the Pb isotope ratios obtained by laser ablation within small areas representative of bone with different Pb exchange rates do not show statistically significant differences. These results are inconsistent with the hypothesis that faulty solder seals in tinned meat were the principle source of Pb in the remains of the expedition personnel.



Similar content being viewed by others
References
O.B. Beattie, J. Geiger, Frozen in Time: The Fate of the Franklin Expedition (Bloomsbury, London, 1987)
F.L. M’Clintock, Fate of Sir John Franklin: The Voyage of the Fox in the Arctic Seas in Search of Franklin and His Companions, 5th edn. (Murray, London, 1881)
W.G. Ross, Arctic 55, 57–69 (2002)
S. Mays, A. Ogden, J. Montgomery, S. Vincent, W. Battersby, G.M. Taylor, J. Archaeol. Sci. 38, 1571–1582 (2011)
R. Amy, R. Bhatnagar, E. Damkjar, O. Beattie, Can. Med. Assoc. J. 135(2), 115–117 (1986)
D.N.H. Notman, L. Anderson, O.B. Beattie, R. Amy, Am. J. Roentgenol. 149, 347–350 (1987)
B. Ranford, Equinox 69, 46–53 (1993)
B. Ranford, Equinox 74, 69–87 (1994)
B.Z. Horowitz, J. Toxicol. 4(6), 841–847 (2003)
W.A. Kowal, P.M. Krahn, O.B. Beattie, Int. J. Environ. Anal. Chem. 35(2), 119–126 (1989)
O.B. Beattie, Muskox 33, 68–77 (1983)
O.B. Beattie, J.M. Savelle, Hist. Archaeol. 17(2), 100–105 (1983)
A. Keenleyside, X. Song, D.R. Chettle, C.E. Webber, J. Archaeol. Sci. 23(3), 461–465 (1996)
W. Kowal, O.B. Beattie, H. Baadsgaard, P.M. Krahn, J. Archaeol. Sci. 18(2), 193–203 (1991)
K.T.H. Farrer, J. Archaeol. Sci. 20(4), 399–409 (1993)
J.J. Pritchard, Bones Carolina Biology Readers, vol. 47 (Oxford University Press, Oxford, 1972), pp. 2–16
R. McNeill Alexander, Human Bones—A Scientific and Pictorial Investigation (PI Press, New York, 2005), pp. 13–15
M.B. Rabinowitz, Environ. Health Perspect. 91, 33–37 (1991)
L.E. Wittmers, A.C. Aufberheide, G. Rapp Jr., Arch. Environ. Health 43(6), 381–391 (1988)
P.R. Flood, P.F. Schmidt, G.R. Wesnberg, H. Gadeholt, Arch. Toxicol. 62, 295–300 (1988)
W.I. Manton, Br. J. Ind. Med. 42, 168–172 (1985)
M.J. Heard, A.C. Chamberlaine, Health Phys. 7(6), 857–865 (1984)
D.R. Smith, J.D. Osterloh, A.R. Flegal, Environ. Health Perspect. 104(1), 60–66 (1996)
R.R. Martin, I.M. Kempson, S.J. Naftel, W.M. Skinner, Chemosphere 58(10), 1385–1390 (2005)
R.W. Elias, P. Hirao, C.C. Patterson, Geochim. Cosmochim. Acta 46, 2561–2580 (1982)
J. Burton, Bone chemistry and trace element analysis, in Biological Anthropology of the Human Skeleton, ed. by M.A. Katzenberg, S.R. Saunders, 2nd edn. (Wiley, New York, 2008), pp. 443–457
A.C. Todd, P.J. Parsons, S. Tang, E.L. Mochier, Environ. Health Perspect. 109(11), 1130–1143 (2001)
W. Kowal, O.B. Beattie, H. Baadsgaard, P.M. Krahn, Nature 343(6256), 319–320 (1990)
F. Facchetti, P. Gaglione, A. Colombo, G. Garibaldi, G. Spallanzani, G. Gilli, Isotopic Pb experiment-status report. EUR 7352, EN. Commission of European Communities, Joint Research Centre, Ispra Establishment (1982)
L.E. Wittmers Jr., A.C. Aufderheide, J.G. Pounds, K.W. Jones, J.L. Angel, Am. J. Phys. Anthropol. 136(4), 379–386 (2008)
T. Swanston, T. Varney, I. Coulthard, R. Feng, B. Brewer, R. Murphy, C. Henning, D. Cooper, J. Archaeol. Sci. 39(7), 2409–2413 (2012)
M. Krachler, J. Zhang, D. Fisher, W. Shotyk, Anal. Chem. 76(18), 5510–5517 (2004)
L. Torrisi, F. Caridi, L. Giuffrida, A. Borrielli A, G. Mondio, in 36th EPS Conference on Plasma Physics, vol. 33E, Sofia, June 29–July 3 (2009), pp. 2–105
J.S. Becker, M.V. Zoriy, C. Pickhardt, N. Palomero-Gallagher, K. Zilles, Anal. Chem. 77(10), 3208–3216 (2005)
J.M. Parish, The Stirrup Court cemetery: an examination of health in nineteenth-century Ontario. Masters thesis, Department of Anthropology, University of Western, Ontario, London, Canada (2000)
S. Gomez, R. Rizzo, M. Pozzi-Mucelli, E. Bonucci, F. Vittur, Bone 25(1), 33–38 (1999)
J.H. Freeland, R.J. Cousins, R. Schwartz, Am. J. Clin. Nutr. 29(7), 745–749 (1976)
P. Poleník, Med. Hypotheses 40(3), 182–185 (1993)
A. Dolphin, S. Naftel, A.J. Nelson, R.R. Martin, C.D. White, J. Arch. Sci. 40, 1778–1786 (2013)
R.R. Martin, S.J. Naftel, A. Nelson, D.W. Sapp III, Can. J. Chem. 85(10), 831–836 (2007)
Acknowledgements
Work supported in part by the U S Department of Energy under contract No. DE-AC02-98CH10886. The US Department of Energy, Office of Science, and Office of Basic Energy Sciences supported use of the National Synchrotron Light Source.
Additional funding was supplied by the Natural Science and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, R.R., Naftel, S., Macfie, S. et al. Pb distribution in bones from the Franklin expedition: synchrotron X-ray fluorescence and laser ablation/mass spectroscopy. Appl. Phys. A 111, 23–29 (2013). https://doi.org/10.1007/s00339-013-7579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-7579-5