Abstract
Objectives
Most previous glaucoma studies with resting-state fMRI have focused on the neuronal activity in the individual structure of the brain, yet ignored the functional communication of anatomically separated structures. The purpose of this study is to investigate the efficiency of the functional communication change or not in glaucoma patients.
Methods
We applied the resting-state fMRI data to construct the connectivity network of 25 normal controls and 25 age-gender-matched primary open angle glaucoma patients. Graph theoretical analysis was performed to assess brain network pattern differences between the two groups.
Results
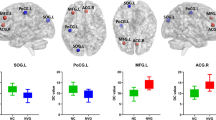
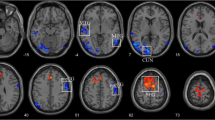
No significant differences of the global network measures were found between the two groups. However, the local measures were radically reorganized in glaucoma patients. Comparing with the hub regions in normal controls’ network, we found that six hub regions disappeared and nine hub regions appeared in the network of patients. In addition, the betweenness centralities of two altered hub regions, right fusiform gyrus and right lingual gyrus, were significantly correlated with the visual field mean deviation.
Conclusions
Although the efficiency of functional communication is preserved in the brain network of the glaucoma at the global level, the efficiency of functional communication is altered in some specialized regions of the glaucoma.
Key Points
• Global topological measures of brain network have no alterations in glaucoma patients.
• Local network measures are radically reorganized in glaucoma patients.
• The alterations of hub regions are found in the glaucoma.
• Betweenness centrality of altered hubs may reflect the glaucoma severity.




Similar content being viewed by others
Abbreviations
- ALFF:
-
Amplitude of low frequency fluctuations
- BC:
-
Betweenness centrality
- BOLD:
-
Blood-oxygen-level dependent
- CDR:
-
Cup-to-disk ratio
- CON:
-
Normal controls
- Deg:
-
Degree
- Eg:
-
Global efficiency
- Eloc:
-
Local efficiency
- FC:
-
Functional connectivity
- FDR:
-
False discovery rate
- MD:
-
Mean deviation
- PAT:
-
Primary open angle glaucoma patients
- POAG:
-
Primary open angle glaucoma
- ReHo:
-
Regional homogeneity
- RNFL:
-
Retinal nerve fiber layer
References
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90:262–267
Whitmore AV, Libby RT, John SW (2005) Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res 24:639–662
Gupta V, Jha R, Rao A, Kong G, Sihota R (2009) The effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucoma. Eur J Ophthalmol 19:435–441
Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM (2007) Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res 26:38–56
Hui ES, Fu QL, So KF, Wu EX (2007) Diffusion tensor MR study of optic nerve degeneration in glaucoma. 2007 Annual International Conference of the Ieee Engineering in Medicine and Biology Society. 1–16:4312–4315
Garaci FG, Bolacchi F, Cerulli A et al (2009) Optic nerve and optic radiation neurodegeneration in patients with glaucoma: In vivo analysis with 3-T diffusion-tensor MR imaging. Radiology 252:496–501
Engelhorn T, Michelson G, Waerntges S, Struffert T, Haider S, Doerfler A (2011) Diffusion tensor imaging detects rarefaction of optic radiation in glaucoma patients. Acad Radiol 18:764–769
Zhang Y, Wan SH, Ge J, Zhang XL (2012) Diffusion tensor imaging reveals normal geniculocalcarine-tract integrity in acquired blindness. Brain Res 1458:34–39
Wang J, Miao W, Li J et al (2015) Automatic segmentation of the lateral geniculate nucleus: Application to control and glaucoma patients. J Neurosci Methods 255:104–114
Chen WW, Wang N, Cai S et al (2013) Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3T MR imaging. Invest Ophthalmol Vis Sci 54:545–554
Gupta N, Yucel YH (2007) Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol 18:110–114
Liu Z, Xu C, Xu Y et al (2010) Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res 182:211–215
Greicius MD, Flores BH, Menon V et al (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437
Cherkassky VL, Kana RK, Keller TA, Just MA (2006) Functional connectivity in a baseline resting-state network in autism. Neuroreport 17:1687–1690
Paakki JJ, Rahko J, Long X et al (2010) Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res 1321:169–179
Liu Y, Yu C, Liang M et al (2007) Whole brain functional connectivity in the early blind. Brain 130:2085–2096
Wang D, Qin W, Liu Y, Zhang Y, Jiang T, Yu C (2014) Altered resting-state network connectivity in congenital blind. Hum Brain Mapp 35:2573–2581
Wang TY, Li Q, Guo MX et al (2014) Abnormal functional connectivity density in children with anisometropic amblyopia at resting-state. Brain Res 1563:41–51
Ding K, Liu Y, Yan X, Lin X, Jiang T (2013) Altered functional connectivity of the primary visual cortex in subjects with amblyopia. Neural Plast 2013(612086):8. doi:10.1155/2013/612086
Lin X, Ding K, Liu Y, Yan X, Song S, Jiang T (2012) Altered spontaneous activity in anisometropic amblyopia subjects: revealed by resting-state fMRI. PLoS One 7(8):e43373. doi:10.1371/journal.pone.0043373
Wang J, Hu L, Li W, Xian J, Ai L, He H (2014) Alternations of functional connectivity in amblyopia patients: a resting-state fMRI study. In: Proc. SPIE 9038, Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, 903809 (March 13, 2014). doi:10.1117/12.2043424
Song Y, Mu K, Wang J et al (2014) Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One 9:e89493
Li T, Liu Z, Li J et al (2015) Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state FMRI study. Invest Ophthalmol Vis Sci 56:322–329
Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400
Zang YF, He Y, Zhu CZ et al (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91
van den Heuvel MP, Hulshoff Pol HE (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20:519–534
Dai H, Morelli JN, Ai F et al (2013) Resting-state functional MRI: functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum Brain Mapp 34:2455–2463
Shu N, Liu Y, Li J, Li Y, Yu C, Jiang T (2009) Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS One 4:e7228
Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA (2014) Brain functional connectivity network breakdown and restoration in blindness. Neurology 83:542–551
Gong GL, Rosa P, Carbonell F, Chen ZJ, He Y, Evans AC (2009) Age- and gender-related differences in the cortical anatomical network. J Neurosci 29:15684–15693
Chen ZJ, He Y, Rosa-Neto P, Gong GL, Evans AC (2011) Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage 56:235–245
He Y, Chen Z, Evans A (2008) Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci 28:4756–4766
Supekar K, Menon V, Rubin D, Musen M, Greicius MD (2008) Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol 4:e1000100
Buckner RL, Sepulcre J, Talukdar T et al (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29:1860–1873
Lo CY, Wang PN, Chou KH, Wang JH, He Y, Lin CP (2010) Diffusion Tensor Tractography Reveals Abnormal Topological Organization in Structural Cortical Networks in Alzheimer’s Disease. J Neurosci 30:16876–16885
Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T et al. (2010) Abnormal cortical networks in mild cognitive impairment and Alzheimer’s Disease. PLoS Comput Biol 6(11):e1001006. doi:10.1371/journal.pcbi.1001006
Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW (2012) Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73:1216–1227
Humphries MD, Gurney K, Prescott TJ (2006) The brainstem reticular formation is a small-world, not scale-free, network. Proc R Soc B Biol Sci 273:503–511
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87(19):198701. doi:10.1103/PhysRevLett.87.198701
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393:440–442
Yan CG, Zang YF (2010) DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069
Achard S, Delon-Martin C, Vertes PE et al (2012) Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A 109:20608–20613
Sporns O, Honey CJ (2006) Small worlds inside big brains. Proc Natl Acad Sci U S A 103:19219–19220
Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006) A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72
Hagmann P, Kurant M, Gigandet X et al (2007) Mapping human whole-brain structural networks with diffusion MRI. PLoS One 2:e597
He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17:2407–2419
Iturria-Medina Y, Sotero RC, Canales-Rodriguez EJ, Aleman-Gomez Y, Melie-Garcia L (2008) Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. Neuroimage 40:1064–1076
Liu Y, Liang M, Zhou Y et al (2008) Disrupted small-world networks in schizophrenia. Brain 131:945–961
Gong GL, He Y, Concha L et al (2009) Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19:524–536
Micheloyannis S, Pachou E, Stam CJ, Vourkas M, Erimaki S, Tsirka V (2006) Using graph theoretical analysis of multi channel EEG to evaluate the neural efficiency hypothesis. Neurosci Lett 402:273–277
Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P (2007) Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex 17:92–99
Stam CJ, de Haan W, Daffertshofer A et al (2009) Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 132:213–224
Wang QF, Su TP, Zhou Y et al (2012) Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage 59:1085–1093
Hanggi J, Wotruba D, Jancke L (2011) Globally altered structural brain network topology in grapheme-color synesthesia. J Neurosci 31:5816–5828
Caeyenberghs K, Leemans A, Heitger MH et al (2012) Graph analysis of functional brain networks for cognitive control of action in traumatic brain injury. Brain 135:1293–1307
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Yuan W, Wade SL, Babcock L (2015) Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum Brain Mapp 36(2):779–792
Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L (2002) The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport 13:321–325
Inman WS (1921) Emotion and eye symptoms. Br J Med Psychol 2:47–67
Schoenberg MJ (1940) Role of states of anxiety in the pathogenesis of primary glaucoma. Arch Ophthalmol 23:76–86
Sykes CS (1949) Role of emotion in glaucoma. Dis Nerv Syst 10:104–107
Ripley HS, Wolff HG (1950) Life situations, emotions, and glaucoma. Psychosom Med 12:215–224
Yochim BP, Mueller AE, Kane KD, Kahook MY (2012) Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma 21:250–254
Acknowledgments
The scientific guarantor of this publication is Huiguang He. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by 863 Projects (2013AA013803), the National Natural Science Foundation of China (61271151, 91520202, 81571649) and the Youth Innovation Promotion Association CAS. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval was given by the Medical Ethics Committee of the Beijing Tongren Hospital. Some study subjects or cohorts have been previously reported in two studies: “Automatic segmentation of the lateral geniculate nucleus: Application to control and glaucoma patients” and “Altered Amplitude of Low Frequency Fluctuation in Primary Open Angle Glaucoma: A Resting State fMRI Study”.
Methodology: retrospective, case–control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.27 mb)
Rights and permissions
About this article
Cite this article
Wang, J., Li, T., Wang, N. et al. Graph theoretical analysis reveals the reorganization of the brain network pattern in primary open angle glaucoma patients. Eur Radiol 26, 3957–3967 (2016). https://doi.org/10.1007/s00330-016-4221-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4221-x