Abstract
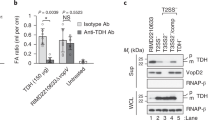
Shigella flexneri secretes an enterotoxic, SPATE family autotransporter (AT), SigA, which has cytopathic activity towards cultured epithelial cells. Its cytopathic activity is due to its ability to degrade the cytoskeletal protein, α-fodrin. The mechanisms by which AT toxins target cells and tissues differ and the details of how SigA acts are not known. In the current study, the determinants of proteolysis and cell-targeting for SigA were determined. We demonstrate that the SigA passenger or α-domain consists of two functionally distinct domains, designated α1 and α2, which are sufficient to specify proteolytic and cell-binding activities, respectively.



Similar content being viewed by others
References
Al-Hasani K, Henderson IR, Sakellaris H, Rajakumar K, Grant T, Nataro JP, Robins-Browne R, Adler B (2000) The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect Immun 68(5):2457–2463
Al-Hasani K, Navarro-Garcia F, Huerta J, Sakellaris H, Adler B (2009) The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS One 4(12):e8223. doi:10.1371/journal.pone.0008223
Amann E, Ochs B, Abel KJ (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69(2):301–315
Benjelloun-Touimi Z, Si Tahar M, Montecucco C, Sansonetti PJ, Parsot C (1998) SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology 144(Pt 7):1815–1822
Brockmeyer J, Spelten S, Kuczius T, Bielaszewska M, Karch H (2009) Structure and function relationship of the autotransport and proteolytic activity of EspP from Shiga toxin-producing Escherichia coli. PLoS One 4(7):e6100. doi:10.1371/journal.pone.0006100
Brunder W, Schmidt H, Karch H (1997) EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol 24(4):767–778
Domingo Meza-Aguilar J, Fromme P, Torres-Larios A, Mendoza-Hernandez G, Hernandez-Chinas U, de Los Arreguin-Espinosa, Monteros RA, Eslava Campos CA, Fromme R (2014) X-ray crystal structure of the passenger domain of plasmid encoded toxin(Pet), an autotransporter enterotoxin from enteroaggregative Escherichia coli (EAEC). Biochem Biophys Res Commun 445(2):439–444. doi:10.1016/j.bbrc.2014.02.016
Dutta PR, Sui BQ, Nataro JP (2003) Structure–function analysis of the enteroaggregative Escherichia coli plasmid-encoded toxin autotransporter using scanning linker mutagenesis. J Biol Chem 278(41):39912–39920. doi:10.1074/jbc.M303595200
Guyer DM, Henderson IR, Nataro JP, Mobley HL (2000) Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol Microbiol 38(1):53–66
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166(4):557–580
Henderson IR, Czeczulin J, Eslava C, Noriega F, Nataro JP (1999) Characterization of pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun 67(11):5587–5596
Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D (2004) Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68(4):692–744. doi:10.1128/MMBR.68.4.692-744.2004
Jenkins J, Pickersgill R (2001) The architecture of parallel beta-helices and related folds. Prog Biophys Mol Biol 77(2):111–175
Khan S, Mian HS, Sandercock LE, Chirgadze NY, Pai EF (2011) Crystal structure of the passenger domain of the Escherichia coli autotransporter EspP. J Mol Biol 413(5):985–1000. doi:10.1016/j.jmb.2011.09.028
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lindberg S, Xia Y, Sonden B, Goransson M, Hacker J, Uhlin BE (2008) Regulatory interactions among adhesin gene systems of uropathogenic Escherichia coli. Infect Immun 76(2):771–780. doi:10.1128/IAI.01010-07
Navarro-Garcia F, Canizalez-Roman A, Sui BQ, Nataro JP, Azamar Y (2004) The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect Immun 72(6):3609–3621. doi:10.1128/IAI.72.6.3609-3621.2004
Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro JP (1999) Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun 67(5):2184–2192
Otto BR, Sijbrandi R, Luirink J, Oudega B, Heddle JG, Mizutani K, Park SY, Tame JR (2005) Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J Biol Chem 280(17):17339–17345. doi:10.1074/jbc.M412885200
Pohlner J, Halter R, Beyreuther K, Meyer TF (1987) Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325(6103):458–462. doi:10.1038/325458a0
Polgar L (2005) The catalytic triad of serine peptidases. Cell Mol Life Sci 62(19–20):2161–2172. doi:10.1007/s00018-005-5160-x
Provence DL, Curtiss R 3rd (1994) Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun 62(4):1369–1380
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67(1):31–40
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Chua, E.G., Al-Hasani, K., Scanlon, M. et al. Determinants of Proteolysis and Cell-Binding for the Shigella flexneri Cytotoxin, SigA. Curr Microbiol 71, 613–617 (2015). https://doi.org/10.1007/s00284-015-0893-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0893-8