Abstract
Purpose
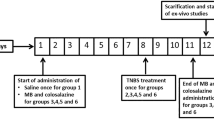
We previously demonstrated the increase of reactive oxygen species (ROS) production and myeloperoxidase (MPO) activity in the small intestine of methotrexate (MTX)-treated rats. In the present study, we investigated the role of ROS modulating intestinal mucosal permeability in this damage.
Method
MTX (20 mg/kg body weight) was administered to rats intravenously. N-Acetylcysteine (NAC; 80 mg/kg body wt), an antioxidant and a precursor of glutathione (GSH) was administered to rats intraperitoneally to investigate the contribution of ROS to the intestinal permeability enhancement. Intestinal permeability was evaluated by determining that of a poorly absorbable marker, fluorescein isothiocyanate-labeled dextran (FD-4; average molecular mass, 4.4 kDa) using the in vitro everted intestine technique. The occurrence of oxidative stress in the small intestine was assayed by measuring chemiluminescence and thiobarbituric acid reactive substances (TBARS) productions in mucosal homogenates of the small intestine.
Results
The mucosal permeability of FD-4 significantly (p < 0.01) increased in MTX-treated rats compared with control rats, as demonstrated by a twofold increase of FD-4 permeation clearance. This suggests an increase in paracellular permeability. Interestingly, the ROS production was observed preceding the increase of paracellular permeability. Treatment with NAC prevented the MTX-induced ROS production and the increase of paracellular permeability.
Conclusions
NAC protected the small intestine of rats from MTX-induced change in paracellular permeability, suggesting that ROS played an important role in the enhanced paracellular permeability.






Similar content being viewed by others
References
Thomas HJ (1987) Searching for magic bullet: early approaches to chemotherapy-antifolate, methotrexate. Cancer Res 47:5528–5536
Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C (1982) Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative therapy. Cancer 49:1221–1230
Ortiz Z, Shea B, Suarez-Almazor ME, Moher D, Wells GA, Tugwell P (1998) The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A metaanalysis of randomized controlled trials. J Rheumatol 25:36–43
Taminiau JA, Gall DG, Hamilton JR (1980) Response of the rat small-intestine epithelium to methotexate. Gut 21:486–492
Pinkerton CR, Cameron CH, Sloan JM, Glasgow JF, Gwevava NJ (1980) Jejunal crypt cell abnormalities associated with methotrexate treatment in children with acute lymphoblatic leukaemia. J Clin Pathol 35:1272–1277
Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK (2004) Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126:796–808
Khan SA, Wingard JR (2001) Infection and mucosal injury in cancer treatment. NCI Monogr 29:31–36
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE (2007) Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG (2002) The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50:53–58
Howarth GS, Cool JC, Bourne AJ, Ballard FJ, Read LC (1998) Insulin-like growth factor-I (IGF-I) stimulates regrowth of the damaged intestine in rats, when administered following, but not concurrent with, methotrexate. Growth Factors 15:279–292
Jahovic N, Sener G, Cevik H, Ersoy Y, Arbak S, Yegen BC (2004) Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct 22:169–178
Tsurui K, Kosakai Y, Horie T, Awazu S (1990) Vitamin A protects the small intestine from methotrexate-induced damage in rats. J Pharmacol Exp Ther 253:1278–1284
Horie T, Nakamaru M, Masubuchi Y (1998) Docosahexaenoic acid exhibits a potent protection of small intestine from methotrexate-induced damage in mice. Life Sci 62:1333–1338
Horie T, Matsumoto H, Kasagi M, Sugiyama A, Kikuchi M, Karasawa C, Awazu S, Itakura Y, Fuwa T (1999) Protective effect of aged garlic extract on the small intestinal damage of rats induced by methotrexate administration. Planta Med 65:545–548
Gao F, Nakamaru M, Masubuchi Y, Horie T (2001) Protective effect of a synthetic analog of prostaglandin E1 on the small intestinal damage induced by the administration of methotexate to rats. J Pharm Sci 90:1040–1048
Miyazono Y, Gao F, Horie T (2004) Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol 39:1119–1127
Gao F, Ueda S, Horie T (2001) Effect of a synthetic analog of prostaglandin E1 on the intestinal mucosa of methotrexate-treated rats. Anticancer Res 21:1913–1917
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Quiles JL, Huertas JR, Battino M, Mataix J, Ramirez-Tortosa MC (2002) Antioxidant nutrients and adriamycin toxicity. Toxicology 180:79–95
Conklin KA (2000) Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 37:1–18
Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H (2001) Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol 174:27–34
Hagiwara SI, Ishii Y, Kitamura S (2000) Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162:225–231
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Nakamaru M, Masubuchi Y, Narimatsu S, Awazu S, Horie T (1998) Evaluation of damaged small intestine of mouse following methotrexate administration. Cancer Chemother Pharmacol 41:98–102
Gao F, Horie T (2002) A synthetic analog of prostaglandin E(1) prevents the production of reactive oxygen species in the intestinal mucosa of methotrexate-treated rats. Life Sci 71:1091–1099
Sheth P, Basuroy S, Li C, Naren AP, Rao RK (2003) Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278:49239–49245
Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A (2002) Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368(Pt 2):471–481
Edens HA, Levi BP, Jaye DL, Walsh S, Reaves TA, Turner JR, Nusrat A, Parkos CA (2002) Neutrophil transepithelial migration: evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J Immunol 169:476–486
Zen K, Parkos CA (2003) Leukocyte-epithelial interactions. Curr Opin Cell Biol 15:557–564
Doluisio JT, Billups NF, Dittert LW, Sugita ET, Swintosky JV (1969) Drug absorption I: an in situ rat gut technique yielding realistic absorption rates. J Pharm Sci 58:1196–1200
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Hartsok A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669
Li T, Ito K, Sumi S-I, Fuwa T, Horie T (2005) Antiapoptosis action of aged garlic extract (AGE) protects epithelial cells from methotrexate induced injury. Gut 54:1819–1820
Li T, Ito K, Sumi S-I, Fuwa T, Horie T (2009) Protective effect of aged garlic extract (AGE) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother Pharmacol 63:873–880
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, T., Miyazono, Y., Ito, K. et al. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol 65, 1117–1123 (2010). https://doi.org/10.1007/s00280-009-1119-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1119-1