Abstract
Over 20 years ago it was realized that the traditional methods of the treatment of injuries to joint components: cartilage, menisci and ligaments, did not give satisfactory results and so there is a need of employing novel, more effective therapeutic techniques. Recent advances in molecular biology, biotechnology and polymer science have led to both the experimental and clinical application of various cell types, adapting their culture conditions in order to ensure a directed differentiation of the cells into a desired cell type, and employing non-toxic and non-immunogenic biomaterial in the treatment of knee joint injuries. In the present review the current state of knowledge regarding novel cell sources, in vitro conditions of cell culture and major important biomaterials, both natural and synthetic, used in cartilage, meniscus and ligament repair by tissue engineering techniques are described, and the assets and drawbacks of their clinical application are critically evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of ageing populations and the consequent increasing incidence of musculoskeletal disorders, attention has recently been focused on the regeneration of joint structures, primarily knee joints. This task requires the employment of novel tissue engineering (TE) techniques necessary to regenerate cartilage, menisci, or ligaments [1, 2]. However, the application of the TE techniques face numerous challenges, including: the appropriate choice of the cell source and the culture methods used for tissue repair, suitable scaffolds and proper bioreactors [3, 4].
Among the cell types, which qualify for the repair of damaged joint structures the most suitable are chondrocytes, fibrochondrocytes, mesenchymal stem cells (MSCs), human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) [5]. The design of a suitable scaffold requires its proper architecture, which ensures optimal conditions for cell growth. When the scaffolds are loaded with the appropriately selected cells, supplied with proper growth factors, subjected to mechanical stimuli in a suitable bioreactor and implanted into the injury site, then the intrinsic healing response can begin [6].
The aim of the present report is to review the current literature on the cell-based regeneration of knee cartilage, menisci and ligaments. The review includes in vitro, ex vivo and in vivo studies as well as clinical trials.
Cell sources
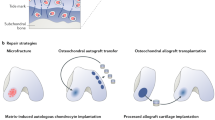
Of the numerous methods available, cell-based techniques are the most advanced approaches. The cells, first considered suitable for transplantation into the lesion site of articular cartilage were autologous chondrocytes [7]. The procedure, which was introduced in an animal model in the early 1980s is called autologous chondrocyte implantation (ACI). In the early 1990s, Brittberg et al. [7] in their initial clinical studies on the effectiveness of ACI, reported good or excellent results in 14 out of 16 patients. Two subsequent clinical trials, conducted in larger groups and longer follow-up terms, showed good-to-excellent results in 76 % [8] and 88 % [9] of patients respectively, indicating that the emerging TE technique would soon replace traditional methods of treatment for cartilage, menisci and ligaments injuries and more suitable cells would be searched for.
Chondrocytes and fibroblasts
Autologous chondrocytes, first used for the repair of articular cartilage injuries, have also been found as a good cell source for the meniscus tear repair [10]. It has been well-established that current methods of meniscus repair are only effective in treating tears located in the vascularized (red-red) zone of the meniscus, while ruptures of the avascular (white-white) zone still present a challenge for cell-based therapies [11]. Since meniscectomy contributes to the premature development of osteoarthritis (OA) [12], research has turned towards meniscus replacement methods that use cell/biomaterial constructs. The potential advantage of chondrocytes over meniscus cells is mainly due to the higher production rate of type II collagen and glycosaminoglycans (GAGs) by chondrocytes [13]. Investigations in animal models have shown that the treatment of a meniscus rupture within the avascular zone with autologous chondrocytes provides a good bonding of the lesion borders [10]. Moreover, it is extremely difficult to obtain a sufficient number of autologous meniscus cells from patients who have already undergone meniscectomy. For these reasons, both autologous and allogenic chondrocytes have been used in the replacement of menisci [14, 15].
Fibroblasts have been used to regenerate ligamentous tissue. Cooper et al. [16] compared four cell sources used for anterior cruciate ligament (ACL) reconstruction. Cells were harvested from the ACL, medial collateral ligament (MCL), Achilles tendon, or patellar tendon. The results showed that fibroblasts from the Achilles and patellar tendons showed the highest proliferation rate. However, only the ACL fibroblasts demonstrated a significantly higher expression of differentiation-specific ligament genes. Ge et al. [17] found that fibroblasts derived from ACLs or MCLs produced type I collagen and α-smooth muscle actin, and did not synthesize type II collagen. In that study, however, fibroblasts showed a lower proliferation rate and ability to survive when compared to mesenchymal stem cells (MSCs). In addition, the use of fibroblasts is troublesome due to several factors, including: (i) reduced number of native cells available for implantation, (ii) cell dedifferentiation in vitro and (iii) the need for an additional operation.
Mesenchymal stem cells
The use of MSCs as an alternative source for therapy is advantageous since the number of cells available for implantation is much higher, cells differentiate in vitro into desired cells and there is no need for a second operation. The major obstacle in the application of MSCs in articular cartilage repair, however, is an uncontrolled process of endochondral ossification, which may occur after cell transplantation [18, 19]. In several studies commonly used bone marrow-derived stem cells or stem cells isolated from various tissues were compared. Pei et al. [20] reported that in MSCs derived from synovial tissue, potential for endochondral ossification was lower than in bone marrow-derived MSCs. When, in an animal model, MSCs were applied to restore full-thickness osteochondral defects, bone marrow, periosteal and synovial MSCs showed a higher potential for lesion repair than adipose-derived and muscle-derived MSCs [21]. Adipose-derived stem cells, have a low potential for differentiation into cartilage because they do not express the transforming growth factor-β (TGF-β) receptor and showed a reduced expression of bone morphogenetic proteins (BMPs) [22]. Nevertheless, in cells isolated from the infrapatellar fat pad, the chondrogenic potential in vitro is similar to that of the MSCs derived from synovium [23, 24]. A disadvantage in using bone marrow-derived MSCs is their lower proliferation rate in the elderly and in the OA patients [25]. The MSCs have been tested in several different animal species [26, 27] as well as in clinical studies in humans [28, 29]. The results of these studies confirm the high chondrogenic potential of MSCs in vivo and the outcomes were similar to those obtained using ACI. In order to reach final conclusions, further research in larger groups of patients is required.
Several researchers have also investigated (in animal models) the effectiveness of autologous MSC transplantation to repair meniscus tears. The results of those experiments showed that MSCs implanted into the avascular area of the meniscal tear proliferate and expand, thus producing large amounts of ECM components and thus accelerating the healing process [30, 31].
MSCs have also been used in ligament regeneration due to the ability to effectively differentiate into the ligament fibroblasts [32]. These cells exhibit the fibroblasts’ phenotype both in vitro [17] and in vivo [33]. Recent advances in elucidating the basic molecular mechanisms underlying cell differentiation and signalling pathways involved in this process, together with the progress in cell homing and implantation techniques could lead to the application of this method in clinical trials.
Human embryonic stem cells
In addition to MSCs, other cell types that present chondrogenic or fibrogenic potential are human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). The first of these, hESCs, derive from human blastocysts and have the potential to differentiate into all three germ layer cell lines. Over the last several years, various protocols were used to differentiate hESCs into chondrocytes [34, 35]. Chondrocytes obtained from hESCs show an immature phenotype; therefore, in order to achieve cell maturation, further refinement of this technique applying special biological and environmental factors as well as biomaterials is required. The potential of hESCs to differentiate into fibrochondrocyte-like cells has been investigated by Hoben et al. [36], who showed that the co-culture of hESCs with chondrocytes or meniscus cells results in a significant increase in tissue-specific collagen production. Results from that study have proven that hESCs are suitable for the repair of the meniscus with fibrocartilage cells. However, because of legal considerations and ethical controversies, the clinical use of hESCs is still under consideration.
Induced pluripotent stem cells
Induced pluripotent stem cells are reprogrammed pluripotent cells, which like hESC, also show the potential to differentiate into three germ layer cell types. Most human iPSCs derive from fibroblasts and they are reprogrammed using the forced expression of transcription factors, which are known oncogenes [37]. For this reason, the clinical application of iPSCs is currently being investigated with great caution, given the high potential for oncogenicity that may result from the use of these factors [38]. However, it should be noticed that the use of iPSCs rather than hESCs avoids an ethical issue since the genetic information contained in iPSCs derives from the patient’s own genome and is also less likely to induce an immune response [39]. The use of iPSCs in TE is an emerging field of research, and may lead to a wide range of alternative methods that can be used in the treatment of injuries of knee joint structures. Preliminary studies have demonstrated that iPSCs derived from mouse fibroblasts differentiate towards chondrocytes, which synthesize ECM components, type II collagen and GAGs. These chondrocytes, in an in vitro model of cartilage defects, integrate with the native tissue when implanted in agarose scaffolds [25].
Culture conditions
The use of cells expanded under conditions that preserve their differentiation potential and phenotype is crucial in the cell-based TE protocols. Cell growth depends on several important factors, including: temperature, pH, oxygen concentration, nutrients, growth factor/cytokine supply, concentration of metabolic waste and culture type (monolayer or 3D) [40]. Typically, cells are cultured at 37 °C, although some reports suggest that oxidative stress can be reduced by culturing MSCs at 32 °C [41]. The pH is normally 7.4 and it decreases when the concentration of metabolic waste products increases in the medium. This parameter should be carefully controlled, since major changes in the pH may be detrimental for the cell culture [42]. Continuous oxygen supply is essential to provide proper dO2 concentration, which decreases as the biomass increases. The atmospheric oxygen level, however, may cause the oxidative stress of the cells, thereby decreasing their viability [43]. It has been reported that MSCs expand more efficiently at 2 % O2 [44, 45], whereas hESCs grow better at 20 to 30 % oxygen levels [46]. Moreover, Foldager et al. [47] demonstrated that oxygen tension (pO2) as low as 9 % in a 3D culture stimulates the expression of genes that encode chondrogenic markers (Sox9, aggrecan and Col2a1).
Nutrient supply is obviously crucial at each stage of cell differentiation. During cell expansion, cell metabolism is mainly based on energy obtained from glycolysis, whereas at the differentiation stage, there is a switch to oxidative phosphorylation as the energy source [48]. It has been well-established that chondrocytes dedifferentiate when cultured in monolayers, whereas they are able to retain their phenotype in the 3D culture [49]. Cell dedifferentiation results in the decreased expression of type II, IX and XI collagens, as well as SOX9 and aggrecan, with a change to “more fibroblastic” collagens such as type I, V collagen and versican [49, 50]. In large 3D constructions, however, the number and metabolism of cells located in the inner part decreases [51]. A lower concentration of cells in the 3D culture would compromise the mechanical strength of the implant. However, this problem can be overcome by applying bioreactors with dynamic fluid circulation, such as a rotating-wall-vessel bioreactor (RWV) or stirred-flask bioreactor [52]. To date, no consensus has been reached concerning optimal cell densities in cell-based therapeutic methods. In different reports cell numbers ranged from 2.5 × 105 [53] to 1 × 107 per cm2 [54]. Another study reported that MSCs require an even higher cell density during the chondrogenesis process [55].
The principal growth factors directing chondrogenesis and fibrogenesis are members of the TGF-β family [56]. Cell expansion and differentiation are also affected by bone morphogenetic proteins (BMPs) and the following growth factors: insulin-like growth factors (IGFs), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and growth and differentiation factors (GDFs) [53, 57].
Cell culture is labour-intensive, time-consuming and expensive. Therefore, efforts have been made to optimize this procedure by using bioreactors to obtain fully controlled, stable, automated cell cultures at a reduced cost [42].
Biomaterials used for cartilage, menisci and ligaments repair
Collagen
Collagen as the major component of cartilage, menisci as well as the ligaments and ECM has long been used in TE as a natural biomaterial [58, 59]. Nowadays, a wide range of collagen-based biomaterials are commercially available. Collagen matrices are characterized by relatively low immunogenicity and a proper structure, which mimic a native tissue environment, thus preventing cell dedifferentiation. However, collagen scaffolds are difficult to handle and sterilization may alter their structure [60]. In collagen matrices type I collagen is commonly used. In vitro studies of type I collagen hydrogel scaffolds has shown that the cells embedded in the hydrogel easily adhere and proliferate well and the MSCs seeded on type I collagen matrix, retain their differentiation potential for a long time. In one of the studies, it was reported that proteoglycan and type II collagen content were similar to that obtained by the same number of cells seeded on clinically used type I/III collagen scaffolds and cultured under the same conditions for 42 days [61]. Yuan et al. [62] demonstrated the immunomodulatory properties of type I collagen hydrogel. Chondrocytes cultured in this gel for 14 days increased the synthesis of both MHC class I and II proteins. These cells produced ECM components, mainly type II collagen and GAGs continuously over the culture period. Gierloff et al. [63] compared the proliferation rate of MSCs and chondrogenesis using various commercially available collagen and hyaluronan scaffolds including: non-cross linked bovine type I matrix, type I/III collagen scaffold, type I collagen sponge and hyaluronic acid gel. Chondrogenesis mediated by MSCs was documented by the elevated expression of the SOX-9 gene, chondroadherin, cartilage oligomeric matrix protein (COMP) and type IX and XI collagens. The cells seeded on the spongy type I collagen scaffold, showed the highest proliferation rate and number of viable cells (over 54 %) compared to the other two collagen matrices and hyaluronan gel.
The collagen-based meniscus implant (CMI) was the first technique of this type used for meniscus regeneration [64]. CMI is a resorbable type I collagen scaffold and was arthroscopically sutured to the remaining parts of the meniscus. This technique is recommended for the repair of the outer parts of the meniscus and is not suitable for the whole tissue [64]. Martinek et al. [65] conducted an in vitro study in an animal model using CMI seeded with autologous fibrochondrocytes. In the experimental group (n = 9), the meniscus tissue was restored after 3 months. In that group, the implants were seeded with cells containing a larger ECM volume, whereas the implants in the control group (non CMI-seeded) were filled with a high number of cells with a small ECM volume. Another preliminary study involving eight patients who were observed for 24 months showed clinical improvement in all patients. Macroscopic evaluation during the second-look arthroscopy revealed tissue regeneration, and histological assessment confirmed the formation of fibrocartilage [66]. Niemeyer et al. [67] performed a long-term clinical study to prove the superiority of standardized collagen membrane over first generation ACI for the treatment of cartilage defects. Another study, conducted in a group of 160 patients who were observed for 12 months, supports previous observations. Although clinical improvement was reported in most patients, the meniscus-like tissue was present in only 50 % of cases [68]. Pabbruwe et al. [69] investigated another commercially available type I/III collagen membrane, used as a carrier for human bone marrow MSCs, in the treatment of meniscus tears of the avascular zone. Cell-loaded and cell-free scaffolds were cultured in vitro on the white zone of ovine menisci. The MSC-loaded constructs integrated well with the host tissue and this group showed significantly better results in tensile strength compared to the acellular constructs.
Gelatin
Gelatin derives from collagen and is a form of denatured collagen fibre. Due to denaturation, gelatine exhibits lower antigenicity than the collagen itself, and is biocompatible and biodegradable. The most commonly used form of gelatine scaffolds is hydrogel, which is created by fibre crosslinking. The fibres are degraded enzymatically, with the degradation time depending on water content [70]. In addition, these scaffolds retain the Arg-Gly-Asp (RGD) sequence, which promotes cell adhesion and proliferation. However, the poor mechanical properties of gelatine gels preclude the use of this polymer alone as a cell-carrier in cartilage, meniscus or ligament regeneration [71]. In order to improve the mechanical strength, in most studies the cross-linked gelatine scaffolds or multilayer constructs were applied [72, 73]. Lien et al. [72] studied a multi-layered gelatine-ceramic construct for osteochondral lesion repair. In the rat model, they showed that a gelatine layer seeded in vitro with chondrocytes led to the development of cartilage tissue in less than four weeks. From the 1st to the 4th week of culture, the GAGs content increased about 20-times, the cells overgrew the scaffold and filled the pores, while only a small number of hypertrophic cells were observed. The same group investigated the influence of gelatine scaffolds pore size on rat chondrocyte growth and synthesis of the ECM components. The authors concluded that these cells secrete more GAGs and overgrow better scaffolds when the pores are larger (from 250 to 500 μm in size). The authors also observed an increase in chondrogenic markers: type I, II and X collagens, as well as aggrecans [74].
For meniscus regeneration, a gelatine-based, three-layered scaffold has been developed by Sarem et al. [75]. These authors seeded heterogeneous gelatine/chitosan matrices with human meniscus cells and, showed increased cell number and proliferation over the culture time. Moreover, they reported that cells seeded on scaffolds with higher gelatine content presented a higher proliferation rate and that the biomaterial was more cytocompatible.
Hyaluronan
Hyaluronan is a polysaccharide polymer most often used for orthopaedic applications in the form of benzyl ester of hyaluronic acid (HA). Its degradation process and HA release has been well-documented [76]. HA is as an important component of GAGs, and it shows major chondrogenic and chondro-protective properties [77, 78], although the clinical application of non-cross-linked hyaluronan-based scaffold is limited due to its weak mechanical strength [79]. Hyaluronan has been used to modify the synthetic PCL (polycaprolactone) scaffold of proper mechanical strength and has been shown to improve the distribution and differentiation of chondrocytes seeded on the PCL scaffolds. Type I and II collagen and aggrecan synthesis was also increased when the HA-modified versus an unmodified PCL were used [80]. More recently, Wu et al. [81] investigated the impact of HA on chondrogenesis in human adipose-derived stem cells (ADSCs). Cells were cultured in a 2D monolayer system using either HA/fibrin or fibrin hydrogels. ECM formation assessment showed that cells seeded on HA/fibrin gel induced higher levels of chondrogenic markers expression (SOX-9, type II collagen and aggrecan). Type II collagen and GAG synthesis on HA/fibrin gel was also superior to that of fibrin gel alone. The authors indicated that induction of chondrogenesis in the HA-enriched scaffold was due to the HA and CD44-cell surface receptor involved in HA interactions.
In the TE of the meniscus, HA was used as a scaffold composite together with gelatine [82], collagen [83, 84] and PCL [85]. In a rabbit model of meniscus defect, MSCs seeded on hyaluronan/gelatine matrices and implanted to the injury site, integrated well with the host tissue, filled the defect and three months, newly formed fibrocartilage, with hyaline-like cartilage zones, was observed [82]. In a more recent study, Zellner et al. [84] compared the effects of repairing meniscus tears in the avascular zone of rabbits using four different treatment methods: untreated tear, suture of the meniscus, a PRP-loaded composite and a scaffold loaded with autologous MSCs. At 12 weeks, no signs of healing were observed in either the untreated or sutured tear groups. The PRP-loaded composite resulted in poor tissue regeneration, while the MSC-seeded scaffold filled the defect with meniscus-like tissue comprising low cell numbers and high type II collagen content. Kon et al. [85] investigated hyaluronan/PCL constructs with improved mechanical properties to assess their value as a cell-carrier of autologous chondrocytes. One-year after surgery, cell-seeded scaffolds showed significantly better fibrocartilage formation when compared with the cell-free implants and the group that underwent meniscectomy.
Fibrin
Fibrin is generated by the proteolytic cleavage of fibrinogen by the enzyme thrombin. Since fibrinogen could be formed from the patients’ fibrin, this biomaterial is immunocompatible and has been widely used in clinical medicine in the form of fibrin glue for cardiovascular, skin, liver, or muscle repair [86]. The mechanical properties and integrity of fibrin hydrogels depend on Ca2+ concentrations and the pH. Similar to gelatine, fibrin scaffolds are characterized by low mechanical strength and rapid degradation. Fibrin-based scaffolds have been used in the TE of cartilage, meniscus and ligaments, although they are usually modified with the addition of more resistant polymers [87]. It has been well-established that fibrin promotes cell proliferation and differentiation [88]. Kreuz et al. [89] investigated the use of scaffold-assisted ACI to repair focal cartilage defects. In that study, 52 patients underwent cartilage repair with autologous chondrocytes embedded in a fibrin scaffold. Four-year clinical observations showed significant improvement in over 80 % of patients, with moderate to complete filling of the lesion observed on MRI.
Fibrin hydrogel has also been investigated in meniscal repair [90]. In that study, chondrocytes embedded in the fibrin scaffold and cultured in vitro bonded to the surrounding tissue and restored the fibrocartilage, while no tissue formation was observed in the cell-free constructs.
Silk fibres
Silk fibres are another emerging and attractive alternative to ECM-derived polymers, mainly due to their outstanding mechanical properties, which are crucial for cartilage, meniscus and ligament regeneration. Silk is a fibrous protein synthesized by several different species of worms, although the best-characterized and most frequently used fibres are produced by the silkworm (Bombyx mori) [91]. Silk fibres show a low immunogenicity and antigenicity, and thus are good candidates for the use as cell-carriers in the TE [92]. In a rabbit model of articular cartilage defect, bone marrow MSCs seeded on silk/chitosan (SF/CS) scaffolds restored the cartilage surface with smooth and well-integrated newly-formed tissue. In contrast, in rabbits who received the cell-free scaffold, only a distinct margin between the native and the regenerated tissue was visible. At 12 weeks after surgery, neither group showed scaffold fibres and no immunogenic reactions occurred [93].
A three-layered silk scaffold has been developed as a cell carrier for meniscal tear repair [94]. The multi-layered structure mimics the fibre alignment of a native human meniscus. The outer parts of silk matrices were seeded with human fibroblasts and the central part was loaded with chondrocytes. At the 28th day of in vitro culture, the cells were evenly distributed across the three layers. GAGs and type I and II collagens were detected in all parts of the construct. The same research group also evaluated the use of MSCs seeded on a multi-layered silk scaffold [95]. In that study, the authors found that MSCs differentiated into chondrogenic lineages after four weeks. The cells stained positive for GAGs and collagen and the differentiated cells showed mature chondrocyte phenotype.
Silk fibres have also been used in numerous in vitro and in vivo studies of ligament regeneration. Liu et al. [96] compared rabbit MSCs and fibroblasts harvested from the ACL and seeded on silk scaffolds. The results of the in vitro study showed that MSCs showed a higher proliferation rate, synthesized more ECM components and exhibited the increased expression of genes encoding type I and II collagen and tenascin-C. The same group investigated the in vivo effects of MSCs seeded onto silk scaffolds and used in ACL reconstruction in the rabbit model [97]. The authors compared two groups, one which received cell-loaded scaffolds and the other (control group) receiving acellular scaffolds. In the 24th week, the cellular scaffolds restored the ACL tissue in the experimental group, while in the control group less of the fibrous tissue formation with visible silk fibres in the regenerated ligament were observed. Histological assessment revealed the intense production of the ECM components, high type I collagen expression and fibroblast-like morphology of the cells. In the control group, all samples stained negative for the ECM components. When converted into a large animal model and evaluated after 24 weeks, the MSCs seeded on the silk scaffold differentiated into fibroblasts and their morphology resembled the native tissue. The follow-up study revealed the complete degradation of silk fibres and the regenerated ACL was able to withstand nearly 52 % of the maximum load compared to the native ligament [33].
Synthetic polymers
Synthetic polymers include poly (lactic acid) (PLA), poly (glycolic acid) (PGA), PLA and PGA copolymer, poly (lactide-co-glycolide (PLGA) and polycaprolactone (PCL).
PLA is used in two isomeric forms: poly L-lactic acid (PLLA) [98] and poly DL-lactic acid (PLDLA) [99], both are biocompatible and biodegradable, with a degradation time of PLA ranging from one to two years [100]. In vivo, PLA is subjected to hydrolysis and the degradation rate depends on the size and shape of implant [101]. Like all synthetic polymers, PLA is easily produced and processed. Polymeric PLDLA-PCL scaffolds were used to repair meniscal tears in a rabbit model [102]. In that study, matrices were seeded with meniscus cells and implanted into the site of the defect. The results of the cell-loaded and cell-free groups were compared at 12 and 24 weeks after implantation. In the 24th week, the tissue that formed in the pre-seeded cell group resembled the native meniscus, while the cell-free scaffolds failed to restore the area of meniscus incision and developed fibrosis at the implantation site. Both the isomeric forms of PLA have been tested for ligament reconstruction. Cells seeded on the PLLA-braided scaffold adhere, proliferate and exhibit ECM component production after three weeks of culture [16].
PGA degrades to glycine, which enters the tricarboxylic acid cycle, and is thus metabolized through the natural metabolic pathways [103]. PGA is an FDA-approved polymer that has long been used in biodegradable sutures. In a pig model study of articular cartilage defect, autologous chondrocytes were seeded and implanted (after two weeks of culture) onto a PGA/PLA scaffold and evaluated after six months [104]. At the 6th-month after the surgery, the cells completely filled the site of the defect with mature cartilage-like tissue. In the newly-formed cartilage, type II collagen and GAG contents were similar to the surrounding tissue. In contrast, the cell-free PGA/PLA construct implantation resulted in the formation of fibrous tissue that did not integrate with the native cartilage, collapsed into the site of the defect and no synthesis of type II collagen or GAGs were detected.
Kang et al. [105] reported the regeneration of rabbit menisci using the PGA/PLGA scaffold seeded with allogenic meniscal cells implanted into the knee joint. At the 10th week, meniscal regeneration was observed and collagen and PG content in the newly-formed tissue was similar to that of native tissue. Saha et al. [106] studied three different cell types cultured under various conditions in vitro and in vivo on the PLGA-based scaffolds. Human bone marrow MSCs, human neonatal chondrocytes and adult chondrocytes were cultured in the chondrogenic medium on the cartilage phase of a commercial osteochondral scaffold. All cell types revealed a high proliferation rate, ECM secretion and cell growth. After the intra-peritoneal implantation of cell-loaded constructs into nude mice, cells formed a cartilage-like tissue. However, in both MSCs and neonatal chondrocytes, some fibrocartilage-like tissue insertions were observed, while the adult chondrocytes managed to maintain their chondrogenic phenotype.
The effect of chondrocyte-seeded PLGA scaffold was examined in meniscal regeneration using porcine meniscal discs implanted in vivo in a heterotopic mouse [107]. In ten out of 12 samples of PLGA cell-loaded constructs, the newly formed tissue filled the entire lesion with a fibrous and cartilaginous tissue, while fibrocartilage was found in seven cases, whereas when acellular scaffolds were used, no signs of healing were observed.
PCL is a soluble polymer with a degradation time of two to three years [108]. A three-layered heterogeneous PCL scaffold that mimics the fibre organization and alignment of native tissue has been developed and one of the studies compared cell attachment, proliferation and differentiation using bovine chondrocytes seeded either on a homogenous PCL scaffold or on the three-layered PCL construct. In that study, no differences in cell adhesion, GAG content, type I and II collagen production were found [109]. Moutos and Guilak [110] studied an anisotropic PCL/fibrin gel scaffold seeded with human ADSCs. Although cells cultured for 28 days under “chondrogenic” conditions synthesized the ECM components, the phenotype of the cells was fibrocartilage-like.
Conclusions
Combining various types of cells, including stem cells, with new biomaterials presents enormous possibilities to engineer the damaged tissues, including knee joint structures. To date, many efforts have been made to overcome the limitations in cell harvesting, in vitro culture and implantation techniques. Novel methods of manufacturing and the emergence of 3D printing have opened new horizons and in the near future the growing expectations of clinical applications will likely appear. Recent developments in cell-based therapeutic methods, especially those using stem cells, ensure a heterogeneous cell environment and provide the biological and mechanical stimuli that mimic the conditions existing in the native tissue. A thorough understanding of the biological processes underlying the TE at both cellular and molecular levels will ensure the safety and effectiveness of these innovations. All these developments, taken together, may in the future, lead to the successful and cost-effective transfer of the TE methods and the use of novel cell/biomaterial constructs from the bench top to the bedside.
References
Pećina M, Vukičević S (2014) Tissue engineering and regenerative orthopaedics (TERO). Int Orthop 38(9):1757–1760. doi:10.1007/s00264-014-2477-9
Legnani C, Ventura A, Terzaghi C et al (2010) Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int Orthop 34(4):465–471. doi:10.1007/s00264-010-0963-2
Wang N, Grad S, Stoddart MJ et al (2014) Particulate cartilage under bioreactor-induced compression and shear. Int Orthop 38(5):1105–1111. doi:10.1007/s00264-013-2194-9
Tarng Y-W, Huang B-F, Su F-C (2012) A novel recirculating flow-perfusion bioreactor for periosteal chondrogenesis. Int Orthop 36(4):863–868. doi:10.1007/s00264-011-1291-x
Oldershaw RA (2012) Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93(6):389–400. doi:10.1111/j.1365-2613.2012.00837.x
Vinatier C, Mrugala D, Jorgensen C et al (2009) Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 27(5):307–314. doi:10.1016/j.tibtech.2009.02.005
Brittberg M, Lindahl A, Nilsson A et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895. doi:10.1056/NEJM199410063311401
Zaslav K, Cole B, Brewster R et al (2008) A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the study of the treatment of articular repair (STAR) clinical trial. Am J Sports Med 37:42–55. doi:10.1177/0363546508322897
Bentley G, Biant LC, Carrington RWJ et al (2003) A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg (Br) 85:223–230
Peretti GM, Gill TJ, Xu J-W et al (2004) Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med 32(1):146–158
Setton LA, Guilak F, Hsu EW, Vail TP (1999) Biomechanical factors in tissue engineered meniscal repair. Clin Orthop Relat Res (367 Suppl): S254–72
Sommerlath KG (1991) Results of meniscal repair and partial meniscectomy in stable knees. Int Orthop 15(4):347–350
Marsano A, Millward-Sadler SJ, Salter DM et al (2007) Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthr Cartil 15:48–58. doi:10.1016/j.joca.2006.06.009
Weinand C, Peretti GM, Adams SB et al (2006) An allogenic cell-based implant for meniscal lesions. Am J Sports Med 34(11):1779–1789. doi:10.1177/0363546506290666
Moran CJ, Atmaca S, Declercq HA et al (2014) Cell distribution and regenerative activity following meniscus replacement. Int Orthop 38(9):1937–1944. doi:10.1007/s00264-014-2426-7
Cooper J Jr, Bailey L, Carter J et al (2006) Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials 27:2747–2754. doi:10.1016/j.biomaterials.2005.12.013
Ge Z, Goh JCH, Lee EH (2005) Selection of cell source for ligament tissue engineering. Cell Transplant 14(8):573–583
Barry F, Boynton RE, Liu B, Murphy JM (2001) Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268(2):189–200. doi:10.1006/excr.2001.5278
Murdoch AD, Grady LM, Ablett MP et al (2007) Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells 25(11):2786–2796. doi:10.1634/stemcells.2007-0374
Pei M, Chen D, Li J, Wei L (2009) Histone deacetylase 4 promotes TGF-β1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 78:260–268. doi:10.1016/j.diff.2009.08.001
Koga H, Muneta T, Nagase T et al (2008) Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 333:207–215. doi:10.1007/s00441-008-0633-5
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295. doi:10.1091/mbc.E02-02-0105
Alegre-Aguarón E, Desportes P, García-Álvarez F et al (2012) Differences in surface marker expression and chondrogenic potential among various tissue-derived mesenchymal cells from elderly patients with osteoarthritis. Cells Tissues Organs 196:231–240. doi:10.1159/000334400
Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ (2012) A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18(11–12):1161–1170. doi:10.1089/ten.TEA.2011.0544
Diekman BO, Christoforou N, Willard VP et al (2012) Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci 109(47):19172–19177. doi:10.1073/pnas.1210422109
Shafiee A, Soleimani M, Chamheidari GA et al (2011) Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res Part A 99(3):467–478. doi:10.1002/jbm.a.33206
Tay LX, Ahmad RE, Dashtdar H et al (2012) Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. Am J Sports Med 40:83–90. doi:10.1177/0363546511420819
Wakitani S, Nawata M, Tensho K et al (2007) Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med 1:74–79. doi:10.1002/term.8
Buda R, Vannini F, Cavallo M et al (2010) Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am 92(Suppl 2):2–11. doi:10.2106/JBJS.J.00813
Izuta Y, Ochi M, Adachi N et al (2005) Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee 12:217–223. doi:10.1016/j.knee.2001.06.001
Nerurkar NL, Han W, Mauck RL, Elliott DM (2011) Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials 32:461–468. doi:10.1016/j.biomaterials.2010.09.015
Hoffmann A, Gross G (2006) Tendon and ligament engineering: from cell biology to in vivo application. Regen Med 1(4):563–574. doi:10.2217/17460751.1.4.563
Fan H, Liu H, Toh SL, Goh JCH (2009) Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 30:4967–4977. doi:10.1016/j.biomaterials.2009.05.048
Toh WS, Lee EH, Cao T (2011) Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev Rep 7(3):544–559. doi:10.1007/s12015-010-9222-6
Oldershaw RA, Baxter MA, Lowe ET et al (2010) Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28(11):1187–1194. doi:10.1038/nbt.1683
Hoben GM, Willard VP, Athanasiou KA (2009) Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev 18(2):283–292. doi:10.1089/scd.2008.0024
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019
Rodolfa K, Di Giorgio FP, Sullivan S (2007) Defined reprogramming: a vehicle for changing the differentiated state. Differentiation 75(7):577–579. doi:10.1111/j.1432-0436.2007.00213.x
Cherry ABC, Daley GQ (2013) Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med 64:277–290. doi:10.1146/annurev-med-050311-163324
Placzek MR, Chung I-M, Macedo HM et al (2009) Stem cell bioprocessing: fundamentals and principles. J R Soc Interface 6(32):209–232. doi:10.1098/rsif.2008.0442
Stolzing A, Scutt A (2006) Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radic Biol Med 41(2):326–338. doi:10.1016/j.freeradbiomed.2006.04.018
Rodrigues CAV, Fernandes TG, Diogo MM et al (2011) Stem cell cultivation in bioreactors. Biotechnol Adv 29(6):815–829. doi:10.1016/j.biotechadv.2011.06.009
Brahimi-Horn MC, Pouysségur J (2007) Oxygen, a source of life and stress. FEBS Lett 581(19):3582–3591. doi:10.1016/j.febslet.2007.06.018
Dos Santos F, Andrade PZ, Boura JS et al (2010) Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol 223(1):27–35. doi:10.1002/jcp.21987
King JA, Miller WM (2007) Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol 11(4):394–398. doi:10.1016/j.cbpa.2007.05.034
Serra M, Brito C, Sousa MFQ et al (2010) Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol 148(4):208–215. doi:10.1016/j.jbiotec.2010.06.015
Foldager CB, Nielsen AB, Munir S et al (2011) Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop 82(2):234–240. doi:10.3109/17453674.2011.566135
Schop D, van Dijkhuizen-Radersma R, Borgart E et al (2010) Expansion of human mesenchymal stromal cells on microcarriers: growth and metabolism. J Tissue Eng Regen Med 4(2):131–140. doi:10.1002/term.224
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30(1):215–224
Stokes DG, Liu G, Dharmavaram R et al (2001) Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J 360(Pt 2):461–470
Häuselmann HJ, Fernandes RJ, Mok SS et al (1994) Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci 107(Pt 1):17–27
Martin I, Wendt D, Heberer M (2004) The role of bioreactors in tissue engineering. Trends Biotechnol 22:80–86. doi:10.1016/j.tibtech.2003.12.001
Gong G, Ferrari D, Dealy CN, Kosher RA (2010) Direct and progressive differentiation of human embryonic stem cells into the chondrogenic lineage. J Cell Physiol 224:664–671. doi:10.1002/jcp.22166
Akmal M, Anand A, Anand B et al (2006) The culture of articular chondrocytes in hydrogel constructs within a bioreactor enhances cell proliferation and matrix synthesis. J Bone Joint Surg (Br) 88:544–553. doi:10.1302/0301-620X.88B4.16498
Johnstone B, Hering TM, Caplan AI et al (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238(1):265–272. doi:10.1006/excr.1997.3858
Augustyniak E, Trzeciak T, Richter M et al (2015) The role of growth factors in stem cell-directed chondrogenesis: a real hope for damaged cartilage regeneration. Int Orthop 39(5):995–1003. doi:10.1007/s00264-014-2619-0
Shen B, Wei A, Whittaker S et al (2009) The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem 109(2):406–416. doi:10.1002/jcb.22412
Iwasa J, Engebretsen L, Shima Y, Ochi M (2009) Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sport Traumatol Arthrosc 17:561–577. doi:10.1007/s00167-008-0663-2
Grassi A, Zaffagnini S, Marcheggiani Muccioli GM et al (2014) Clinical outcomes and complications of a collagen meniscus implant: a systematic review. Int Orthop 38(9):1945–1953. doi:10.1007/s00264-014-2408-9
Friess W (1998) Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm 45(2):113–136
Steck E, Bertram H, Walther A et al (2010) Enhanced biochemical and biomechanical properties of scaffolds generated by flock technology for cartilage tissue engineering. Tissue Eng Part A 16:3697–3707. doi:10.1089/ten.tea.2009.0817
Yuan T, Zhang L, Li K et al (2014) Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J Biomed Mater Res Part B Appl Biomater 102(2):337–344. doi:10.1002/jbm.b.33011
Gierloff M, Nitsche T, Adam-Klages S et al (2014) In vitro comparison of different carrier materials with rat bone marrow MSCs. Clin Oral Investig 18(1):247–259. doi:10.1007/s00784-013-0956-9
Scotti C, Hirschmann MT, Antinolfi P et al (2013) Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater 26:150–170
Martinek V, Ueblacker P, Bräun K et al (2006) Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg 126:228–234. doi:10.1007/s00402-005-0025-1
Rodkey WG, Steadman JR, Li ST (1999) A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Relat Res (367 Suppl):S281–92
Niemeyer P, Salzmann G, Feucht M et al (2014) First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop 38:2065–2070. doi:10.1007/s00264-014-2368-0
Rodkey WG, DeHaven KE, Montgomery WH et al (2008) Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am 90(7):1413–1426. doi:10.2106/JBJS.G.00656
Pabbruwe MB, Kafienah W, Tarlton JF et al (2010) Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials 31(9):2583–2591. doi:10.1016/j.biomaterials.2009.12.023
Malafaya PB, Silva GA, Reis RL (2007) Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev 59:207–233. doi:10.1016/j.addr.2007.03.012
Zhao W, Jin X, Cong Y et al (2013) Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol 88:327–339. doi:10.1002/jctb.3970
Lien S-M, Chien C-H, Huang T-J (2009) A novel osteochondral scaffold of ceramic–gelatin assembly for articular cartilage repair. Mater Sci Eng C 29:315–321. doi:10.1016/j.msec.2008.07.017
Xing Q, Zhao F, Chen S et al (2010) Porous biocompatible three-dimensional scaffolds of cellulose microfiber/gelatin composites for cell culture. Acta Biomater 6(6):2132–2139. doi:10.1016/j.actbio.2009.12.036
Lien S-M, Ko L-Y, Huang T-J (2009) Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater 5(2):670–679. doi:10.1016/j.actbio.2008.09.020
Sarem M, Moztarzadeh F, Mozafari M, Shastri VP (2013) Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater Sci Eng C 33:4777–4785. doi:10.1016/j.msec.2013.07.036
Laurent TC, Fraser JR (1992) Hyaluronan. FASEB J 6(7):2397–2404. doi:10.1016/S0740-8315(82)80016-8
Yoo HS, Lee EA, Yoon JJ, Park TG (2005) Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 26:1925–1933. doi:10.1016/j.biomaterials.2004.06.021
Grigolo B, De Franceschi L, Roseti L et al (2005) Down regulation of degenerative cartilage molecules in chondrocytes grown on a hyaluronan-based scaffold. Biomaterials 26(28):5668–5676. doi:10.1016/j.biomaterials.2005.02.030
Solchaga LA, Temenoff JS, Gao J et al (2005) Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthr Cartil 13(4):297–309. doi:10.1016/j.joca.2004.12.016
Lebourg M, Rochina JR, Sousa T et al (2013) Different hyaluronic acid morphology modulates primary articular chondrocyte behavior in hyaluronic acid-coated polycaprolactone scaffolds. J Biomed Mater Res Part A 101A:518–527. doi:10.1002/jbm.a.34349
Wu S-C, Chen C-H, Chang J-K et al (2013) Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J Appl Physiol 114(11):1610–1618. doi:10.1152/japplphysiol.01132.2012
Angele P, Johnstone B, Kujat R et al (2008) Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res Part A 85(2):445–455. doi:10.1002/jbm.a.31480
Zellner J, Mueller M, Berner A et al (2010) Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res Part A 94(4):1150–1161. doi:10.1002/jbm.a.32796
Zellner J, Hierl K, Mueller M et al (2013) Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J Biomed Mater Res Part B Appl Biomater 101(7):1133–1142. doi:10.1002/jbm.b.32922
Kon E, Filardo G, Tschon M et al (2012) Tissue engineering for total meniscal substitution: animal study in sheep model—results at 12 months. Tissue Eng Part A 18:1573–1582. doi:10.1089/ten.tea.2011.0572
Ahmed TAE, Dare EV, Hincke M (2008) Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 14(2):199–215. doi:10.1089/ten.teb.2007.0435
Eyrich D, Brandl F, Appel B et al (2007) Long-term stable fibrin gels for cartilage engineering. Biomaterials 28(1):55–65. doi:10.1016/j.biomaterials.2006.08.027
Bensaïd W, Triffitt JT, Blanchat C et al (2003) A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 24(14):2497–2502
Kreuz PC, Müller S, Freymann U et al (2011) Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am J Sports Med 39:1697–1705. doi:10.1177/0363546511403279
Scotti C, Pozzi A, Mangiavini L et al (2009) Healing of meniscal tissue by cellular fibrin glue: an in vivo study. Knee Surg Sport Traumatol Arthrosc 17:645–651. doi:10.1007/s00167-009-0745-9
Vepari C, Kaplan DL (2007) Silk as a biomaterial. Prog Polym Sci 32(8–9):991–1007. doi:10.1016/j.progpolymsci.2007.05.013
Meinel L, Kaplan DL (2012) Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev 64(12):1111–1122. doi:10.1016/j.addr.2012.03.016
Deng J, She R, Huang W et al (2013) A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. J Mater Sci Mater Med 24(8):2037–2046. doi:10.1007/s10856-013-4944-z
Mandal BB, Park S-H, Gil ES, Kaplan DL (2011) Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials 32(2):639–651. doi:10.1016/j.biomaterials.2010.08.115
Mandal BB, Park S-H, Gil ES, Kaplan DL (2011) Stem cell-based meniscus tissue engineering. Tissue Eng Part A 17(21–22):2749–2761. doi:10.1089/ten.tea.2011.0031
Liu H, Fan H, Toh SL, Goh JCH (2008) A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials 29(10):1443–1453. doi:10.1016/j.biomaterials.2007.11.023
Fan H, Liu H, Wong EJW et al (2008) In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 29(23):3324–3337. doi:10.1016/j.biomaterials.2008.04.012
Nishimoto H, Kokubu T, Inui A et al (2012) Ligament regeneration using an absorbable stent-shaped poly-l-lactic acid scaffold in a rabbit model. Int Orthop 36(11):2379–2386. doi:10.1007/s00264-012-1660-0
Ikeda R, Fujioka H, Nagura I et al (2009) The effect of porosity and mechanical property of a synthetic polymer scaffold on repair of osteochondral defects. Int Orthop 33(3):821–828. doi:10.1007/s00264-008-0532-0
Garlotta D (2001) A literature review of poly ( lactic acid ). J Polym Environ 9:63–84
Armentano I, Bitinis N, Fortunati E et al (2013) Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog Polym Sci 38:1720–1747. doi:10.1016/j.progpolymsci.2013.05.010
Esposito AR, Moda M, Cattani SMDM et al (2013) PLDLA/PCL-T scaffold for meniscus tissue engineering. Biores Open Access 2(2):138–147. doi:10.1089/biores.2012.0293
An YH, Woolf SK, Friedman RJ (2000) Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials 21(24):2635–2652. doi:10.1016/S0142-9612(00)00132-0
Cui L, Wu Y, Cen L et al (2009) Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials 30:2683–2693. doi:10.1016/j.biomaterials.2009.01.045
Kang S-W, Sun-Mi S, Jae-Sun L et al (2006) Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J Biomed Mater Res Part A 77A:659–671. doi:10.1002/jbm.a.30579
Saha S, Kirkham J, Wood D et al (2013) Informing future cartilage repair strategies: a comparative study of three different human cell types for cartilage tissue engineering. Cell Tissue Res 352:495–507. doi:10.1007/s00441-013-1586-x
Yoo JJ, Bichara DA, Zhao X et al (2011) Implant-assisted meniscal repair in vivo using a chondrocyte-seeded flexible PLGA scaffold. J Biomed Mater Res Part A 99(1):102–108. doi:10.1002/jbm.a.33168
Doppalapudi S, Jain A, Khan W, Domb AJ (2014) Biodegradable polymers-an overview. Polym Adv Technol 25:427–435. doi:10.1002/pat.3305
McCullen SD, Autefage H, Callanan A et al (2012) Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng Part A 18(19–20):2073–2083. doi:10.1089/ten.tea.2011.0606
Moutos FT, Guilak F (2010) Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng Part A 16(4):1291–1301. doi:10.1089/ten.TEA.2009.0480
Acknowledgments
This work was supported by the National Science Centre [grant number 2012/07/E/NZ3/01819].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Trzeciak, T., Richter, M., Suchorska, W. et al. Application of cell and biomaterial-based tissue engineering methods in the treatment of cartilage, menisci and ligament injuries. International Orthopaedics (SICOT) 40, 615–624 (2016). https://doi.org/10.1007/s00264-015-3099-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3099-6