Abstract
Purpose
To establish PET as a tool for in-vivo quantification and monitoring of intramyocardially transplanted stem cells after labelling with FDG in mice with induced myocardial infarction.
Methods
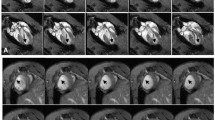
After inducing myocardial infarction in C57BL/6 mice, murine embryonic stem cells were labelled with FDG and transplanted into the border zone of the infarction. Dynamic PET scans were acquired from 25 to 120 min after transplantation, followed by a scan with 20 MBq FDG administered intravenously for anatomical landmarking. All images were reconstructed using the OSEM 3D and MAP reconstruction algorithms. FDG data were corrected for cellular tracer efflux and used as marker for cellular retention. FACS analysis of transplanted cells expressing enhanced green fluorescent protein was performed to validate the PET data.
Results
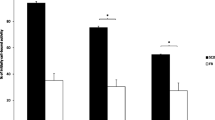
We observed a rapid loss of cells from the site of transplantation, followed by stable retention over 120 min. Amounts of retention were 5.3 ± 1.1 % at 25 min, 5.0 ± 0.9 % at 60 min and 5.7 ± 1.2 % at 120 min. FACS analysis showed a high correlation without significant differences between the groups (P > 0.05). FDG labelling did not have any adverse effects on cell proliferation or differentiation.
Conclusion
Up-to-date imaging is a powerful method for tracking and quantifying intramyocardially transplanted stem cells in vivo in the mouse model. This revealed a massive cell loss within minutes, and thereafter a relatively stable amount of about 5 % remaining cells was observed. Our method may become crucial for further optimization of cardiac cell therapy in the widely used mouse model of infarction.







Similar content being viewed by others
References
Grady KL, Dracup K, Kennedy G, Moser DK, Piano M, Stevenson LW, et al. Team management of patients with heart failure: a statement for healthcare professionals from The Cardiovascular Nursing Council of the American Heart Association. Circulation. 2000;102:2443–56.
Rischpler C, Nekolla S, Schwaiger M. PET and SPECT in heart failure. Curr Cardiol Rep. 2013;15:337.
Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: cellular biology and potential applications. Annu Rev Physiol. 1999;61:219–42.
Frantz RP, Olson LJ. Recipient selection and management before cardiac transplantation. Am J Med Sci. 1997;314:139–52.
Boyle A, Colvin-Adams M. Recipient selection and management. Semin Thorac Cardiovasc Surg. 2004;16:358–63.
Hunt SA. Current status of cardiac transplantation. JAMA. 1998;280:1692–8.
Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56.
Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8.
Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Nussbaum J, Minami E, Laflamme MA, Virag JAI, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57.
Cai W, Zhang Y, Kamp TJ. Imaging of induced pluripotent stem cells: from cellular reprogramming to transplantation. Am J Nucl Med Mol Imaging. 2011;1:18–28.
David R, Stieber J, Fischer E, Brunner S, Brenner C, Pfeiler S, et al. Forward programming of pluripotent stem cells towards distinct cardiovascular cell types. Cardiovasc Res. 2009;84:263–72.
Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17.
Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–27.
David R, Groebner M, Franz W-M. Magnetic cell sorting purification of differentiated embryonic stem cells stably expressing truncated human CD4 as surface marker. Stem Cells. 2005;23:477–82.
Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16.
Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81.
Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26.
Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamäki R, et al. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiosphere-derived cells. J Nucl Cardiol. 2011;18:443–50.
Lautamäki R, Terrovitis J, Bonios M, Yu J, Tsui BM, Abraham MR, et al. Perfusion defect size predicts engraftment but not early retention of intra-myocardially injected cardiosphere-derived cells after acute myocardial infarction. Basic Res Cardiol. 2011;106:1379–86.
Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–44.
Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841.
Brunner S, Winogradow J, Huber BC, Zaruba MM, Fischer R, David R, et al. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. FASEB J. 2009;23:351–61.
Deindl E, Zaruba M-M, Brunner S, Huber B, Mehl U, Assmann G, et al. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–8.
van Laake LW, Passier R, Monshouwer-Kloots J, Nederhoff MG, Ward-van Oostwaard D, Field LJ, et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat Protoc. 2007;2:2551–67.
Mannheim JG, Judenhofer MS, Schmid A, Tillmanns J, Stiller D, Sossi V, et al. Quantification accuracy and partial volume effect in dependence of the attenuation correction of a state-of-the-art small animal PET scanner. Phys Med Biol. 2012;57:3981–93.
Botti C, Negri DR, Seregni E, Ramakrishna V, Arienti F, Maffioli L, et al. Comparison of three different methods for radiolabelling human activated T lymphocytes. Eur J Nucl Med. 1997;24:497–504.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Ieda M, Fu J-D, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86.
Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–22.
Hong J, He H, Weiss ML. Derivation and characterization of embryonic stem cells lines derived from transgenic Fischer 344 and Dark Agouti rats. Stem Cells Dev. 2012;21:1571–86.
Kawamata M, Ochiya T. Gene-manipulated embryonic stem cells for rat transgenesis. Cell Mol Life Sci. 2011;68:1911–5.
Visser EP, Disselhorst JA, Brom M, Laverman P, Gotthardt M, Oyen WJG, et al. Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med. 2009;50:139–47.
Cheng J-CK, Shoghi K, Laforest R. Quantitative accuracy of MAP reconstruction for dynamic PET imaging in small animals. Med Phys. 2012;39:1029–41.
Disselhorst JA, Brom M, Laverman P, Slump CH, Boerman OC, Oyen WJG, et al. Image-quality assessment for several positron emitters using the NEMA NU 4-2008 standards in the Siemens Inveon small-animal PET scanner. J Nucl Med. 2010;51:610–7.
Blackwood KJ, Lewden B, Wells RG, Sykes J, Stodilka RZ, Wisenberg G, et al. In vivo SPECT quantification of transplanted cell survival after engraftment using (111)In-tropolone in infarcted canine myocardium. J Nucl Med. 2009;50:927–35.
Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–61.
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24.
Goddard G, Martin JC, Naivar M, Goodwin PM, Graves SW, Habbersett R, et al. Single particle high resolution spectral analysis flow cytometry. Cytometry A. 2006;69:842–51.
Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154–61.
Wolfs E, Struys T, Notelaers T, Roberts SJ, Sohni A, Bormans G, et al. 18F-FDG labeling of mesenchymal stem cells and multipotent adult progenitor cells for PET imaging: effects on ultrastructure and differentiation capacity. J Nucl Med. 2013;54:447–54.
Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, et al. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5.
Acknowledgments
We thank Christiane Groß, Barbara Markieton and Judith Arcifa for expert technical assistance. This work was supported by the FöFoLe Program of the LMU Munich (C.L. and W.M.F.), the BMBF (01GN0960 to R.D. and W.M.F.), and the Deutsche Forschungsgemeinschaft (DA 1296/2-1 to R.D. and FR 705/14-2 to W.M.F.). W.M.F. is the principal investigator of the Munich Heart Alliance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cajetan Lang and Sebastian Lehner contributed equally to this work.
Marcus Hacker and Robert David share the senior authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 27 kb)
(MOV 11032 kb)
ESM Video 2
(AVI 16851 kb)
Rights and permissions
About this article
Cite this article
Lang, C., Lehner, S., Todica, A. et al. Positron emission tomography based in-vivo imaging of early phase stem cell retention after intramyocardial delivery in the mouse model. Eur J Nucl Med Mol Imaging 40, 1730–1738 (2013). https://doi.org/10.1007/s00259-013-2480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2480-1