Abstract
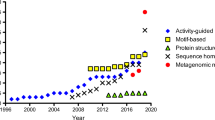
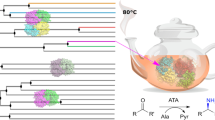
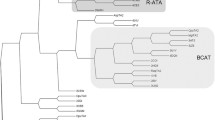
Compared to (S)-selective amine transaminase ((S)-AT), the (R)-selective counterpart ((R)-AT) has been less studied. As such, a simplified “Motif Sequence Blast” search (Höhne et al. Nat Chem Biol 6:807–813, 2010) was carried out to identify new (R)-ATs from the protein databases. The combined conserved sequence motifs of (R)-ATs based on the previous in silico method of predicting (R)-selective amine transaminase were used as the template sequence for BLASTP search at default settings in NCBI, and six candidate sequences were identified. These putative (R)-AT genes were synthesized and overexpressed in Escherichia coli. Among them, five new (R)-ATs were expressed as soluble protein and showed unusual substrate specificity and high stereoselectivity. Furthermore, several unnatural amino acids, such as d-alanine, d-2-aminobutyric acid, and d-norvaline, were synthesized via the (R)-AT-catalyzed amino transfer reaction to the corresponding keto acids. Optically pure (S)-amines were also obtained by kinetic resolution of racemic amines catalyzed with these new (R)-ATs. Therefore, the Motif Sequence Blast search offers a quick and effective method for in silico identification of new (R)-ATs, and the newly identified (R)-ATs are attractive additions to the toolbox of (R)-ATs for further study and industrial application.


Similar content being viewed by others
References
Aguilar M-I, Purcell AW, Devi R, Lew R, Rossjohn J, Smith AI, Perlmutter P (2007) β-Amino acid-containing hybrid peptides—new opportunities in peptidomimetics. Org Biomol Chem 5:2884–2890
Bea H-S, Park H-J, Lee S-H, Yun H (2011) Kinetic resolution of aromatic β-amino acids by ω-transaminase. Chem Commun 47:5894–5896
Benítez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Guillemette T, van Peij NN, Goosen T, Lanthaler K, Robson GD, van den Hondel CA, Stam H, Archer DB (2007) Genomic analysis of the secretion stress response in the enzyme-producing cell factory Aspergillus niger. BMC Genomics 8:158
Höhne M, Bornscheuer UT (2009) Biocatalytic routes to optically active amines. ChemCatChem 1:42–51
Höhne M, Schätzle S, Jochens H, Robins K, Bornscheuer UT (2010) Rational assignment of key motifs for function guides in silico enzyme identification. Nat Chem Biol 6:807–813
Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH (2009) Structural and biological mimicry of protein surface recognition by α/β-peptide foldamers. Proc Natl Acad Sci U S A 106:14751–14756
Hwang BY, Park HY, Lee BS (2008) Identification of ω-aminotransferase from Caulobacter crescentus and sitedirected mutagenesis to broaden substrate specificity. J Microbiol Biotechnol 18:48–54
Iwasaki A, Matsumoto K, Hasegawa J, Yasohara Y (2012) A novel transaminase,(R)-amine: pyruvate aminotransferase, from Arthrobacter sp. KNK168 (FERM BP-5228): purification, characterization, and gene cloning. Appl Microbiol Biotechnol 93:1563–1573
Kim J, Kyung D, Yun H, Cho B-K, Seo J-H, Cha M, Kim B-G (2007) Cloning and characterization of a novel β-transaminase from Mesorhizobium sp. Strain LUK: a new biocatalyst for the synthesis of enantiomerically pure β-amino acids. Appl Environ Microbiol 73:1772–1782
Kohls H, Steffen-Munsberg F, Höhne M (2014) Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr Opin Chem Biol 19:180–192
Koszelewski D, Tauber K, Faber K, Kroutil W (2010) ω-Transaminases for the synthesis of non-racemic α-chiral primary amines. Trends Biotechnol 28:324–332
Łyskowski A, Gruber C, Steinkellner G, Schürmann M, Schwab H, Gruber K, Steiner K (2014) Crystal structure of an (R)-selective ω-transaminase from Aspergillus terreus. PLoS One 9:e87350
Ma L-J, Van Der Does HC, Borkovich KA, Coleman JJ, Daboussi M-J, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B (2010a) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464:367–373
Ma SK, Gruber J, Davis C, Newman L, Gray D, Wang A, Grate J, Huisman GW, Sheldon RA (2010b) A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem 12:81–86
Mathew S, Yun H (2012) ω-Transaminases for the production of optically pure amines and unnatural amino acids. ACS Catal 2:993–1001
Mutti FG, Fuchs CS, Pressnitz D, Turrini NG, Sattler JH, Lerchner A, Skerra A, Kroutil W (2012) Amination of ketones by employing two new (S)-selective ω-transaminases and the His-tagged ω-TA from Vibrio fluvialis. Eur J Org Chem 2012:1003–1007
Otsuka M, Masuda T, Haupt A, Ohno M, Shiraki T, Sugiura Y, Maeda K (1990) Synthetic studies on antitumor antibiotic, bleomycin. 27. Man-designed bleomycin with altered sequence specificity in DNA cleavage. J Am Chem Soc 112:838–845
Park E-S, Dong J-Y, Shin J-S (2014) Active site model of (R)-selective ω-transaminase and its application to the production of D-amino acids. Appl Microbiol Biotechnol 98:651–660
Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Jarvis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J (2010) Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329:305–309
Schätzle S, Steffen-Munsberg F, Thontowi A, Höhne M, Robins K, Bornscheuer UT (2011) Enzymatic asymmetric synthesis of enantiomerically pure aliphatic, aromatic and arylaliphatic amines with (R)-selective amine transaminases. Adv Synth Catal 353:2439–2445
Shin J-S, Kim B-G (2002) Exploring the active site of amine: pyruvate aminotransferase on the basis of the substrate structure-reactivity relationship: how the enzyme controls substrate specificity and stereoselectivity. J Org Chem 67:2848–2853
Shin J-S, Yun H, Jang J-W, Park I, Kim B-G (2003) Purification, characterization, and molecular cloning of a novel amine: pyruvate transaminase from Vibrio fluvialis JS17. Appl Microbiol Biotechnol 61:463–471
Stephens OM, Kim S, Welch BD, Hodsdon ME, Kay MS, Schepartz A (2005) Inhibiting HIV fusion with a β-peptide foldamer. J Am Chem Soc 127:13126–13127
Thomsen M, Skalden L, Palm GJ, Hohne M, Bornscheuer UT, Hinrichs W (2014) Crystallographic characterization of the (R)-selective amine transaminase from Aspergillus fumigatus. Acta Crystallogr D Biol Crystallogr 70:1086–1093
Urano N, Fukui S, Kumashiro S, Ishige T, Kita S, Sakamoto K, Kataoka M, Shimizu S (2011) Directed evolution of an aminoalcohol dehydrogenase for efficient production of double chiral aminoalcohols. J Biosci Bioeng 111:266–271
Wu J, Chen X, Guo R, C-h Y, Chan AS (2003) Ru-catalyzed highly enantioselective hydrogenation of β-alkyl-substituted β-(acylamino) acrylates. J Org Chem 68:2490–2493
Yamada Y, Iwasaki A, Kizaki N, Matsumoto K, Ikenaka Y, Ogura M, Hasegawa J (2000) Process for producing optically active amino compounds. EP Patent 0,987,332
Yun H, Lim S, Cho B-K, Kim B-G (2004) ω-amino acid: pyruvate transaminase from Alcaligenes denitrificans Y2k-2: a new catalyst for kinetic resolution of β-amino acids and amines. Appl Environ Microbiol 70:2529–2534
Acknowledgments
This work was financially supported by National Basic Research Program of China (973 Program, no. 2011CB710801), the National Natural Science Foundation of China (grant no. 21072151), and the CAS Special Grant for Postgraduate Research, Innovation and Practice (grant no. Y2J8041021). We thank Professor Xiaoping Yi (Chinese Academy of Tropical Agricultural Sciences) for supplying the strain Fusarium oxysporum Fo5176.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 209 kb)
Rights and permissions
About this article
Cite this article
Jiang, J., Chen, X., Zhang, D. et al. Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast. Appl Microbiol Biotechnol 99, 2613–2621 (2015). https://doi.org/10.1007/s00253-014-6056-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6056-1