Abstract
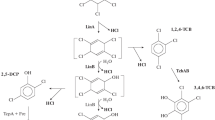
A bacterial strain producing a β-lactam antibiotic acylase, able to hydrolyze ampicillin to 6-aminopenicillanic acid more efficiently than penicillin G, was isolated from soil and characterized. The isolate was identified as Achromobacter sp. using the phenotypic characteristics, composition of cellular fatty acids and 16S rRNA gene sequence. The enzyme synthesis was fully induced by phenylacetic acid (PAA) at a concentration of 2 g l−1. PAA at concentrations up to 12 g l−1 had no negative effect on the specific activity of acylase and biomass production, but slowed down the specific growth rate. Benzoic or 4-hydroxyphenylacetic acids can also induce synthesis of the enzyme. The inducers were metabolized in all cases. Acylase activity in cell-free extracts was determined with various substrates; ampicillin, cephalexin and amoxicillin were hydrolyzed 1.5- and 2-times faster than penicillin G. A high stability of acylase activity was observed over a wide range of pH (5.0–8.5) and at temperatures above 55°C.





Similar content being viewed by others
References
Alvaro G, Fernandez-Lafuente R, Rosell CM, Blanco RM, García-Lopez JL, Guisán JM (1992) Penicillin G acylase from Kluyvera citrophila; new choice as industrial enzyme. Biotechnol Lett 14:285–290
Arroyo M, de la Mata I, Acebal C, Castillón MP (2003) Biotechnological applications of penicillin acylases: state-of-the-art. Appl Microbiol Biotechnol 60:507–514
Baker WL (1992) Co-existence of β-lactamase and penicillin acylase in bacteria; detection and quantitative determination of enzyme activities. J Appl Bacteriol 73:14–22
Balasingham K, Warburton D, Dunnill P, Lilly MD (1972) The isolation and kinetics of penicillin amidase from Escherichia coli. Biochim Biophys Acta 276:250–256
Chiang C, Bennet RE (1967) Purification and properties of penicillin amidase from Bacillus megaterium. J Bacteriol 93:302–306
Díaz E, Ferrández A, Prieto MA, García JL (2001) Biodegradation of aromatic compounds by Escherichia coli. Microbiol Mol Biol Rev 65:523–569
Felsenstein J (1993) PHYLIP (Phylogeny Inference Package), version 3.5.1. Department of Genetics, University of Washington, Seattle, Wash.
Fernández-Lafuente R, Hernándéz-Jústiz O, Mateo C, Terreni M, Fernández-Lorente G, Moreno MA, Alonso J, García-López JL, Guisan JM (2001) Biotransformations catalyzed by multimeric enzymes: stabilization of tetrameric ampicillin acylase permits the optimization of ampicillin synthesis under dissociation conditions. Biomacromolecules 2:95–104
Ferrández A, Miňanbres B, García B, Olivera ER, Luengo JM, García JL, Diaz E (1998) Catabolism of phenylacetic acid in Escherichia coli. Characterization of new aerobic hybrid pathway. J Biol Chem 273:25974–25986
Fujii T, Matsumoto K, Watanabe T (1976) Enzymatic synthesis of cephalexin. Process Biochem 11:21–24
García B, Olivera ER, Miňanbres B, Fernández-Valverde M, Caňedo, Prieto MSA, García JL, Martínez M, Luengo JM (1999) Novel biodegradative aromatic plastics from a bacterial source. Genetic and biochemical studies on a route of the phenylacetyl-CoA catabolon. J Biol Chem 274:29228–29241
García B, Olivera ER, Miňambres B, Carnicero D, Muniz C, Naharro G, Luengo JM (2000) Phenylacetyl-coenzyme A is the true inducer of the phenylacetic acid catabolon pathway in Pseudomonas putida U. Appl Environ Microbiol 66:4575–4578
Ignatova Z, Taruttis S, Kasche V (2000) Role of the intracellular proteolysis in the production of the periplasmic penicillin amidase in Escherichia coli. Biotechnol Lett 22:1727–1732
Illanes A, Acevedo F, Gentina JC, Reyes I, Torres R, Cartagena O, Ruiz A, Vásquez M (1994) Production of penicillin acylase from Bacillus megaterium in complex and defined media. Process Biochem 29:263–270
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic press, New York, pp 21–132
Kawamori M, Hashimoto Y, Katsumata R, Okachi R, Takayama K (1983) Enzymatic production of amoxycillin by β-lactamase-deficient mutant of Pseudomonas melanogenum KY 3987. Agric Biol Chem 47:2503–2509
Kim DJ, Byun SM (1990) Purification and properties of ampicillin acylase from Pseudomonas melanogenum. Biochim Biophys Acta 1040:12–18
Krieg NR, Holt JG (eds) (1984) Bergey's manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore
Kutzbach K, Rauenbusch E (1974) Preparation and general properties of crystalline penicillin acylase from E. coli ATCC 11105. Hoppe-Seyler's Z Physiol Chem 355:45–53
Levitov MM, Klapovskaja KI, Kleiner GI (1967) Induced acylase in Escherichia coli. Mikrobiologiya 36:912–917
Luengo JM, García JL, Olivera ER (2001) The phenylacetyl-CoA catabolon: a complex catabolic unit with broad biotechnological applications. Mol Microbiol 39:1434–1442
Maidak BL, Cole JR, Parker CT Jr, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Nara T, Misawa M, Okachi R, Yamamoto M (1971) Enzymatic synthesis of d(−)-α-aminobenzylpenicillin. Part I. Selection of penicillin acylase-producing bacteria. Agric Biol Chem 35:1676–1682
Nichols NN, Harwood CS (1995) Repression of 4-hydroxybenzoate transport and degradation by benzoate: a new layer of regulatory control in the Pseudomonas putida β-ketoadipate pathway. J Bacteriol 177:7033–7040
O'Connor KE, Witholt B, Duetz W (2001) p-Hydroxyphenylacetic acid metabolism in Pseudomonas putida F6. J Bacteriol 183:928–933
Ohashi H, Katsuda Z, Nagashima M, Kamei T, Yano M (1989) Expression of the Arthrobacter viscosus penicillin G acylase gene in Escherichia coli and Bacillus subtilis. Appl Environ Microbiol 55:1351–1356
Okachi R, Kato F, Miyamura Y, Nara T (1973) Selection of Pseudomonas melanogenum KY 3987 as a new ampicillin-producing bacteria. Agric Biol Chem 37:1953–1957
Olivera ER, Reglero A, Martínez-Blanco H, Fernández-Medarde A, Moreno MA, Luengo JM (1994) Catabolisms of aromatics in Pseudomonas putida U. Formal demonstration that phenylacetic acid and 4-hydroxyphenylacetic acid are catabolized by two unrelated pathways. Eur J Biochem 221:375–381
Olivera ER, Miňambres B, García B, Muniz C, Moreno MA, Ferrández A, Díaz E, García JL, Luengo JM (1998) Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc Natl Acad Sci USA 95:6419–6424
Parmar A, Kumar H, Marwaha SS, Kennedy JF (2000) Advances in enzymatic transformation of penicillins to 6-aminopenicillanic acid (6-APA). Biotechnol Adv 18:289–301
Plháčková K, Kyslík P, Bečka S, Sobotková Ir (2002) The strain of microorganism Comamonas testosteroni CCM 4824. Czech patent No. 291 154
Polderman-Tijmes JJ, Jekel PA, de Vries EJ, van Merode AEJ, Floris R, van der Laan JM, Sonke T, Janssen DB (2002) Cloning, sequence analysis, and expression in Escherichia coli of the gene encoding an α-amino acid ester hydrolase from Acetobacter turbidans. Appl Environ Microbiol 68:211–218
Prieto MA, García JL (1997) Identification of the 4-hydroxyphenylacetate transport gene of Escherichia coli W: construction of a highly sensitive cellular biosensor. FEBS Lett 414:293–297
Prieto MA, Díaz E, García JL (1996) Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J Bacteriol 178:111–120
Quax WJ (1991) Penicillin G acylase, a gene encoding the same and a method for production of this enzyme. European Patent Application EP 0 453 047 A1
Ramos-González MI, Godoy P, Alaminos M, Ben-Bassat A, Ramos JL (2001) Physiological characterization of Pseudomonas putida DOT-T1E tolerance to p-hydroxybenzoate. Appl Environ Microbiol 67:4338–4341
Roa A, Castillón MP, Goble ML, Virden R, García JL (1995) New insights on the specificity of penicillin acylase. Biochem Biophys Res Commun 206:629–636
Robak M, Szewczuk A (1981) Penicillin amidase from Proteus rettgeri. Acta Biochim Pol 28:275–284
Ryu YW, Ryu DDY (1988) Semisynthetic β-lactam antibiotic synthetizing enzyme from Acetobacter turbidans: catalytic properties. Enzyme Microb Technol 10:239–245
Savidge TA, Cole M (1975) Penicillin acylase (bacterial). In: Mosbach K (ed) Methods in enzymology, vol 43. Academic Press, New York, pp 705–721
Schleissner C, Olivera ER, Fernández-Valverde M, Luengo JM (1994) Aerobic catabolism of phenylacetic acid in Pseudomonas putida U: biochemical characterization of a specific phenylacetic acid transport system and formal demonstration that phenylacetyl-coenzyme A is a catabolic intermediate. J Bacteriol 176:7667–7676
Škrob F, Bečka S, Plháčková K, Fotopulosová V, Kyslík P (2003) Novel penicillin G acylase from Achromobacter sp. CCM 4824. Enzyme Microb Technol 32:738–744
Szentirmai A (1964) Production of penicillin acylase. Appl Microbiol 12:185–187
Szentirmai A (1965) Properties of penicillin acylase isolated from Escherichia coli. Acta Microbiol Acad Sci Hung 12:395–405
Takahashi T, Yamazaki Y, Kato K (1974) Substrate specificity of an α-amino ester acid hydrolase produced by Acetobacter turbidans A.T.C.C 9325. Biochem J 137:497–503
Valle F, Balbás P, Merino E, Bolivar F (1991) The role of penicillin amidases in nature and industry. Trends Biochem 16:36–40
Yabuuchi E, Kawamura Y, Kosako Y, Ezaki T (1989) Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) Comb. Nov., Achromobacter piechaudii (Kiredjian et al.) Comb. Nov., and Achromobacter xylosoxidans subsp. denitrificans (Rüger and Tan) Comb. Nov. Microbiol Immunol 42:429–438
Acknowledgement
This work was supported by Institutional Research Concept No. AV0Z5020903.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plháčková, K., Bečka, S., Škrob, F. et al. Isolation and characterization of a new strain of Achromobacter sp. with β-lactam antibiotic acylase activity. Appl Microbiol Biotechnol 62, 507–516 (2003). https://doi.org/10.1007/s00253-003-1353-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1353-0