Abstract
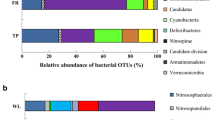
We compared the microbial community composition in soils from the Brazilian Amazon with two contrasting histories; anthrosols and their adjacent non-anthrosol soils of the same mineralogy. The anthrosols, also known as the Amazonian Dark Earths or terra preta, were managed by the indigenous pre-Colombian Indians between 500 and 8,700 years before present and are characterized by unusually high cation exchange capacity, phosphorus (P), and calcium (Ca) contents, and soil carbon pools that contain a high proportion of incompletely combusted biomass as biochar or black carbon (BC). We sampled paired anthrosol and unmodified soils from four locations in the Manaus, Brazil, region that differed in their current land use and soil type. Community DNA was extracted from sampled soils and characterized by use of denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism. DNA bands of interest from Bacteria and Archaea DGGE gels were cloned and sequenced. In cluster analyses of the DNA fingerprints, microbial communities from the anthrosols grouped together regardless of current land use or soil type and were distinct from those in their respective, paired adjacent soils. For the Archaea, the anthrosol communities diverged from the adjacent soils by over 90%. A greater overall richness was observed for Bacteria sequences as compared with those of the Archaea. Most of the sequences obtained were novel and matched those in databases at less than 98% similarity. Several sequences obtained only from the anthrosols grouped at 93% similarity with the Verrucomicrobia, a genus commonly found in rice paddies in the tropics. Sequences closely related to Proteobacteria and Cyanobacteria sp. were recovered only from adjacent soil samples. Sequences related to Pseudomonas, Acidobacteria, and Flexibacter sp. were recovered from both anthrosols and adjacent soils. The strong similarities among the microbial communities present in the anthrosols for both the Bacteria and Archaea suggests that the microbial community composition in these soils is controlled more strongly by their historical soil management than by soil type or current land use. The anthrosols had consistently higher concentrations of incompletely combusted organic black carbon material (BC), higher soil pH, and higher concentrations of P and Ca compared to their respective adjacent soils. Such characteristics may help to explain the longevity and distinctiveness of the anthrosols in the Amazonian landscape and guide us in recreating soils with sustained high fertility in otherwise nutrient-poor soils in modern times.









Similar content being viewed by others
References
Baldock JA, Smernik RJ (2002) Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org Geochem 33:1093–1109
Borga P, Nilsson M, Tunlid A (1994) Bacterial communities in peat in relation to botanical composition as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 26:841–848
Borneman J, Triplett E (1997) Molecular microbial diversity in soils from eastern Amazonia: evidence for unusual microorganisms and microbial population shifts associated with deforestation. Appl Environ Microbiol 63:2647–2653
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Buckley DH, Schmidt TM (2001) Environmental factors influencing the distribution of rRNA from Verrucomicrobia in soil. FEMS Microbiol Ecol 35:105–112
Carney K, Matson P (2005) Plant communities, soil microorganisms, and soil carbon cycling: does altering the world belowground matter to ecosystem functioning? Ecosystems 8:928–940
Carney KM, Matson PA (2006) The influence of tropical plant diversity and composition on soil microbial communities. Microb Ecol 52:226–238
Cleveland CC, Townsend AR, Schmidt SK, Constance BC (2003) Soil microbial dynamics and biogeochemistry in tropical forests and pastures, southwestern Costa Rica. Ecol Appl 13:314–326
Culman SW, Duxbury JM, Lauren JG, Thies JE (2006) Microbial community response to soil solarization in Nepal's rice-wheat cropping system. Soil Biol Biochem 38:3359–3371
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
DeLuca TH, Sala A (2006) Frequent fire alters nitrogen transformations in ponderosa pine stands of the inland northwest. Ecology 87:2511–2522
Eden MJ, Bray W, Herrera L, McEwan C (1984) Terra preta soils and their archaeological context in the caqueta basin of southeast Colombia. Am Antiquity 49:125–140
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Gauch H Jr (1992) Statistical analysis of regional yield trials: AMMI analysis of factorial designs. Elsevier Science Publishers, Amsterdam
Gauch H Jr, Furnas R (1991) Statistical analysis of yield trials with MATMODEL. Agron J 83:916–920
Glaser B (2007) Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Philos Trans R Soc B-Biol Sci 362:187–196
Glaser B, Guggenberger G, Haumaier L, Zech W (2001) Persistence of soil organic matter in archaeological soils (Terra Preta) of the Brazilian Amazon region. In: Rees B, Ball B, Campbell C, Watson C (eds) Sustainable management of soil organic matter. CAB International, Wallingford, pp 190–194
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralisation. Organ Geochem 35:823–830
Henrot J, Robertson GP (1994) Vegetation removal in two soils of the humid tropics—effect on microbial biomass. Soil Biol Biochem 26:111–116
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Ishii T, Kadoya K (1994) Effects of charcoal as a soil conditioner on citrus growth and vesicular arbuscular mycorrhizal development. J Jpn Soc Hortic Sci 63:529–535
Kim JS, Sparovek G, Longo RM, De Melo WJ, Crowley D (2007) Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol Biochem 39:684–690
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lane DJ (1991) 16S/23S rRNA sequencing. In: Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–117
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387
Lehmann J, da Silva JP, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357
Lehmann J, Kern D, German L, McCann J, Van Coimbra MG, Moreira A (2003) Soil fertility and production potential. In: Lehmann J, Kern D, Glaser B, Woods WI (eds) Amazonian dark earths: origin, properties and management. Kluwer Academic Publishers, Boston, pp 105–124
Leininger S et al (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Liang B et al (2006) Black Carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liang B et al (2008) Stability of biomass-derived black carbon in soils. Geochim Cosmochim Acta 72:6069–6078
Luizao RCC, Bonde TA, Rosswall T (1992) Seasonal variation of soil microbial biomass—the effects of clearfelling a tropical rainforest and establishment of pasture in the central amazon. Soil Biol Biochem 24:805–813
Marris E (2006) Putting the carbon back: black is the new green. Nature 442:624–626
Moeseneder MM, Arrieta JM, Muyzer G, Winter C, Herndl GJ (1999) Optimization of terminal-restriction fragment length polymorphism analysis for complex marine bacterioplankton communities and comparison with denaturing gradient gel electrophoresis. Appl Environ Microbiol 65:3518–3525
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S ribosomal rna. Appl Environ Microbiol 59:695–700
Neill BE (2007) Microbial communities in Amazonian dark earth soils analyzed by culture-based and molecular approaches. In: Crop and Soil Sciences vol. M.S. Cornell University, Ithaca, NY, p 76
Neves EG, Petersen JB, Bartone RN, Augusto Da Silva C (2003) Historical and socio-cultural origins of Amazonian dark earths. In: Lehmann J, Kern D, Glaser B, Woods WI (eds) Amazonian dark earths: origin, properties, management. Kluwer Academic Publishers, London, pp 29–50
Nusslein K, Tiedje JM (1999) Soil bacterial community shift correlated with change from forest to pasture vegetation in a tropical soil. Appl Environ Microbiol 65:3622–3626
O’Neill B, Grossman JM, Tsai MT, Gomes JE, Lehmann J, Peterson J, Neves E, Thies JE (2009) Bacterial community composition in Brazilian anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58:23–35
Ovreas L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373
Paterson E, Gebbing T, Abel C, Sim A, Telfer G (2007) Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol 173:600–610
Pesaro M, Widmer F (2002) Identification of novel Crenarchaeota and Euryarchaeota clusters associated with different depth layers of a forest soil. FEMS Microbiol Ecol 42:89–98
Petersen JB (2001) Gift from the past: terra preta and prehistroic amerindian occupation in Amazonia. In: McEwan C (ed) Unknown Amazonia. British Museum, London, pp 86–105
Pietikainen J, Kiikkila O, Fritze H (2000) Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 89:231–242
Preston CM, Schmidt MWI (2006) Black (pyrogenic) carbon: a synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 3:397–420
Robertson CE, Harris JK, Spear JR, Pace NR (2005) Phylogenetic diversity and ecology of environmental Archaea. Cur Opin Microbiol 8:638–642
Rondon MA, Lehmann J, Ramirez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708
Sangwan P, Chen XL, Hugenholtz P, Janssen PH (2004) Chthoniobacter flavus gen. nov., sp. nov., the first pure-culture representative of subdivision two, spartobacteria classis nov., of the phylum Verrucomicrobia. Appl Environ Microbiol 70:5875–5881
Schmidt MWI, Kogel-Knabner I (2002) Organic matter in particle-size fractions from A and B horizons of a Haplic Alisol. Eur J Soil Sci 53:383–391
Schmidt MWI, Skjemstad JO, Jager C (2002) Carbon isotope geochemistry and nanomorphology of soil black carbon: black chernozemic soils in central Europe originate from ancient biomass burning. Global Biogeochem Cycles 16:70.71–70.78
Tanahashi T, Murase J, Matsuya K, Hayashi M, Kimura M, Asakawa S (2005) Bacterial communities responsible for the decomposition of rice straw compost in a Japanese rice paddy field estimated by DGGE analysis of amplified 16S rDNA and 16S rRNA fragments. Soil Sci Plant Nutr 51:351–360
Thies JE, Rillig MC (2008) Characteristics of biochar: biological properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management. Earthscan, Dunstan House, London
Thies JE, Suzuki K (2003) Amazonian dark earths: biological measurements. In: Lehmann J, Kern D, Glaser B, Woods WI (eds) Amazonian dark earths: origin, properties and management. Kluwer Academic Publishers, Norwell, pp 287–332
Tipper JC (1979) Rarefaction and rarefiction—use and abuse of a method in paleoecology. Paleobiology 5:423–434
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grossman, J.M., O’Neill, B.E., Tsai, S.M. et al. Amazonian Anthrosols Support Similar Microbial Communities that Differ Distinctly from Those Extant in Adjacent, Unmodified Soils of the Same Mineralogy. Microb Ecol 60, 192–205 (2010). https://doi.org/10.1007/s00248-010-9689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9689-3