Abstract
Introduction
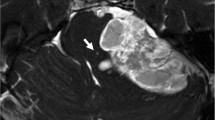
With reported characteristic MR features, it is difficult to differentiate vestibular schwannomas (VSs) from cerebellopontine angle (CPA) meningiomas (CPAMs) in some cases. This study aimed to evaluate vestibular signal intensity changes in patients with VS and those with CPAM on three-dimensional fast imaging employing steady-state acquisition (3D-FIESTA), and to test the effectiveness of the signal intensity change to differentiate these two common CPA tumors.
Methods
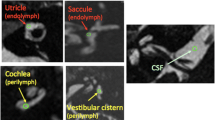
We retrospectively reviewed 21 patients with unilateral VS, six patients with unilateral CPAM, and 25 control subjects. Setting regions of interest in the bilateral vestibules and cerebellar white matter on 3D-FIESTA, we compared the ratio of the signal intensity (SIR) of the vestibule to that of the cerebellar white matter (SIRv) among the VS, CPAM, and control subject groups. We also compared the ratio of SIRv on the affected side (a-SIRv) to that on the unaffected side (AURv) between the VS and CPAM.
Results
The a-SIRv in the VS group was significantly lower than the overall SIRv in the control subjects (pre-contrast, P < 0.001; post-contrast, P < 0.001) and the a-SIRv in the CPAM group (pre-contrast, P = 0.001; post-contrast, P = 0.001). The AURv in the VS group was significantly lower than that in the CPAM groups (pre-contrast, P < 0.001; post-contrast, P < 0.001).
Conclusion
Decreased vestibular signal intensity on the affected side on 3D-FIESTA was observed in patients with VS, but not in those with CPAM or in normal subjects. The signal intensity change has the potential to be used in differentiating VS from CPAM.





Similar content being viewed by others
References
Zamani AA (2000) Cerebellopontine angle tumors: role of magnetic resonance imaging. Top Magn Reson Imaging 11:98–107
Voss NF, Vrionis FD, Heilman CB, Robertson JH (2000) Meningiomas of the cerebellopontine angle. Surg Neurol 53:439–446, discussion 446–47
Nakamura M, Roser F, Dormiani M, Matthies C, Vorkapic P, Samii M (2005) Facial and cochlear nerve function after surgery of cerebellopontine angle meningiomas. Neurosurgery 57:77–89, discussion 90
Gerganov V, Bussarsky V, Romansky K, Popov R, Djendov S, Dimitrov I (2003) Cerebellopontine angle meningiomas. Clinical features and surgical treatment. J Neurosurg Sci 47:129–135
Sanna M, Russo A, Taibah A, Falcioni M, Agarwal M (2004) Enlarged translabyrinthine approach for the management of large and giant acoustic neuromas: a report of 175 consecutive cases. Ann Otol Rhinol Laryngol 113:319–328
Nitz WR (2002) Fast and ultrafast non-echo-planar MR imaging techniques. Eur Radiol 12:2866–2882
Schmitz B, Hagen T, Reith W (2003) Three-dimensional true FISP for high-resolution imaging of the whole brain. Eur Radiol 13:1577–1582
Mikami T, Minamida Y, Yamaki T, Koyanagi I, Nonaka T, Houkin K (2005) Cranial nerve assessment in posterior fossa tumors with fast imaging employing steady-state acquisition (FIESTA). Neurosurg Rev 28:261–266
Xie T, Zhang XB, Yun H, Hu F, Yu Y, Gu Y (2011) 3D-FIESTA MR images are useful in the evaluation of the endoscopic expanded endonasal approach for midline skull-base lesions. Acta Neurochir 153:12–18
Yamamoto J, Kakeda S, Takahashi M, Aoyama Y, Soejima Y, Saito T, Akiba D, Korogi Y, Nishizawa S (2011) Dural attachment of intracranial meningiomas: evaluation with contrast-enhanced three-dimensional fast imaging with steady-state acquisition (FIESTA) at 3 T. Neuroradiology 53:413–423
Rogg JM, Ahn SH, Tung GA, Reinert SE, Norén G (2005) Prevalence of hydrocephalus in 157 patients with vestibular schwannoma. Neuroradiology 47:344–351
Bamford CR, Labadie EL (1976) Reversal of dementia in normotensive hydrocephalus after removal of cauda equina tumor. J Neurosurg 45:104–107
O’Connor AF, France MW, Morrison AW (1981) Perilymph total protein levels associated with cerebello-pontine angle lesions. Am J Otol 2:193–195
Naganawa S, Satake H, Iwano S, Sone M, Nakashima T (2008) Communication between cochlear perilymph and cerebrospinal fluid through the cochlear modiolus visualized after intratympanic administration of Gd-DTPA. Radiat Med 26:597–602
Kawai H, Naganawa S, Ishihara S, Sone M, Nakashima T (2010) MR imaging of the cochlear modiolus after intratympanic administration of Gd-DTPA. Magn Reson Med Sci 9:23–29
Okamoto K, Ito J, Furusawa T, Sakai K, Horikawa S, Tokiguchi S (1998) MRI of enlarged endolymphatic sacs in the large vestibular aqueduct syndrome. Neuroradiology 40:167–172
Okamoto K, Ito J, Furusawa T, Sakai K, Tokiguchi S (1997) Large vestibular aqueduct syndrome with high CT density and high MR signal intensity. AJNR Am J Neuroradiol 18:482–484
World Health Organization. Grades of Hearing impairment. http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html. Accessed 11 June 2012
Silverstein H (1971) Inner ear fluid proteins in acoustic neuroma, Meniere’s disease, and otosclerosis. Ann Otol Rhinol Laryngol 80:27–35
Thomsen J, Saxtrup O, Tos M (1982) Quantitated determination of proteins in perilymph in patients with acoustic neuromas. ORL J Otorhinolaryngol Relat Spec 44:61–65
Rasmussen N, Bendtzen K, Thomsen J, Tos M (1984) Antigenicity and protein content of perilymph in acoustic neuroma patients. Acta Otolaryngol 97:502–508
Buckingham RA, Valvassori GE (2001) Inner ear fluid volumes and the resolving power of magnetic resonance imaging: can it differentiate endolymphatic structures? Ann Otol Rhinol Laryngol 110:113–117
Bhadelia RA, Tedesco KL, Hwang S, Erbay SH, Lee PH, Shao W, Heilman C (2008) Increased cochlear fluid-attenuated inversion recovery signal in patients with vestibular schwannoma. AJNR Am J Neuroradiol 29:720–723
Yamazaki M, Naganawa S, Kawai H, Nihashi T, Fukatsu H, Nakashima T (2009) Increased signal intensity of the cochlea on pre- and post-contrast enhanced 3D-FLAIR in patients with vestibular schwannoma. Neuroradiology 51:855–863
Lee IH, Kim HJ, Chung WH, Kim E, Moon JW, Kim ST, Kim KH, Jeon P, Byun HS (2010) Signal intensity change of the labyrinth in patients with surgically confirmed or radiologically diagnosed vestibular schwannoma on isotropic 3D fluid-attenuated inversion recovery MR imaging at 3 T. Eur Radiol 20:949–957
Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E (2001) Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol 22:87–94
Gopen Q, Rosowski JJ, Merchant SN (1997) Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res 107:9–22
Rask-Andersen H, Schrott-Fischer A, Pfaller K, Glueckert R (2006) Perilymph/modiolar communication routes in the human cochlea. Ear Hear 27:457–465
Juliano AFT, Maya MM, Lo WWM, Kovanlikaya I (2011) Temporal bone tumors and cerebellopontine angle lesions. In: Som PM, Curtin HD (eds) Head and neck imaging, 5th edn. Mosby, St. Louis, pp 1263–1407
Lalwani AK, Jackler RK (1993) Preoperative differentiation between meningioma of the cerebellopontine angle and acoustic neuroma using MRI. Otolaryngol Head Neck Surg 109:88–95
Ryoo JW, Na DG, Woo JY, Park K, Kim HD, Choi DS, Byun HS (2001) Investigation of juxtasellar and cerebellopontine angle meningiomas and neurogenic tumours: two-phase helical CT. Neuroradiology 43:637–643
Thamburaj K, Radhakrishnan VV, Thomas B, Nair S, Menon G (2008) Intratumoral microhemorrhages on T2*-weighted gradient-echo imaging helps differentiate vestibular schwannoma from meningioma. AJNR Am J Neuroradiol 29:552–557
Paz-Fumagalli R, Daniels DL, Millen SJ, Meyer GA, Thieu TM (1991) Dural “tail” associated with an acoustic schwannoma in MR imaging with gadopentetate dimeglumine. AJNR Am J Neuroradiol 12:1206
Kutcher TJ, Brown DC, Maurer PK, Ghaed VN (1991) Dural tail adjacent to acoustic neuroma: MR features. J Comput Assist Tomogr 15:669–670
Okamoto K, Furusawa T, Ishikawa K, Sasai K, Tokiguchi S (2006) Focal T2 hyperintensity in the dorsal brain stem in patients with vestibular schwannoma. AJNR Am J Neuroradiol 27:1307–1311
Yamazaki M, Naganawa S, Kawai H, Nihashi T, Nakashima T (2010) Signal alteration of the cochlear perilymph on 3 different sequences after intratympanic Gd-DTPA administration at 3 tesla: comparison of 3D-FLAIR, 3D-T1-weighted imaging, and 3D-CISS. Magn Reson Med Sci 9:65–71
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishikawa, K., Haneda, J. & Okamoto, K. Decreased vestibular signal intensity on 3D-FIESTA in vestibular schwannomas differentiating from meningiomas. Neuroradiology 55, 261–270 (2013). https://doi.org/10.1007/s00234-012-1100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-012-1100-2