Abstract
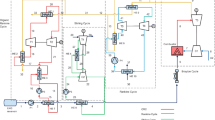
The effective utilization of the cryogenic exergy associated with liquefied natural gas (LNG) vaporization is important. In this paper, a novel combined power cycle is proposed which utilizes LNG in different ways to enhance the power generation of a power plant. In addition to the direct expansion in the appropriate expander, LNG is used as a low-temperature heat sink for a middle-pressure gas cycle which uses nitrogen as working fluid. Also, LNG is used to cool the inlet air of an open Brayton gas turbine cycle. These measures are accomplished to improve the exergy recovery of LNG. In order to analyze the performance of the system, the influence of several key parameters such as pressure ratio of LNG turbine, ratio of the mass flow rate of LNG to the mass flow rate of air, pressure ratio of different compressors, LNG pressure and inlet pressure of nitrogen compressor, on the thermal efficiency and exergy efficiency of the offered cycle is investigated. Finally, the proposed combined cycle is optimized on the basis of first and second laws of thermodynamics.














Similar content being viewed by others
Abbreviations
- m :
-
Mass flow rate (kg/s)
- h :
-
Enthalpy (kJ/kg)
- LHV:
-
Lower heating value (kJ/kg)
- T :
-
Temperature (K)
- e :
-
Specific exergy (kJ/kg)
- E :
-
Exergy (kW)
- LA:
-
Ratio of LNG mass flow rate to air mass flow rate (−)
- NA:
-
Ratio of nitrogen mass flow rate to air mass flow rate (−)
- r p :
-
Pressure ratio (−)
- P :
-
Pressure (MPa)
- s :
-
Specific entropy (kJ/kg-K)
- I :
-
Irreversibility (kW)
- W :
-
Power (kW)
- η:
-
Efficiency (%)
- CMB:
-
Combustion
- inlet:
-
Inlet of device
- outlet:
-
Outlet of device
- c :
-
Cold stream
- h :
-
Hot stream
- loss:
-
Loss
- 0:
-
Ambient
- fuel:
-
Fuel
- in:
-
Input
- out:
-
Output
- th:
-
Thermal
- ex:
-
Exergy
- s :
-
Stack
References
Lu T, Wang KS (2009) Analysis and optimization of a cascading power cycle with liquefied natural gas (LNG) cold energy recovery. Appl Therm Eng 29:1478–1484
Trevisani L, Fabbri M, Negrini F, Ribani PL (2007) Advanced energy recovery systems from liquid hydrogen. Energy Convers Manag 48:146–154
Kaneko K, Ohtani K, Tsujikawa Y, Fujii S (2004) Utilization of the cryogenic exergy of LNG by a mirror gas-turbine. Appl Energy 79:355–369
Kim TS, Ro ST (2000) Power augmentation of combined cycle power plants using cold energy of liquefied natural gas. Energy 25:841–856
Dispenza C, Dispenza G, Rocca VL, Panno G (2009) Exergy recovery during LNG regasification: electric energy production—part one. Appl Therm Eng 29:380–387
Dispenza C, Dispenza G, Rocca VL, Panno G (2009) Exergy recovery during LNG regasification: electric energy production—part two. Appl Therm Eng 29:388–399
Shi X, Che D (2009) A combined power cycle utilizing low-temperature waste heat and LNG cold energy. Energy Convers Manag 50:567–575
Engineering Equation Solver, Commercial and Professional Versions 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salimpour, M.R., Zahedi, M.A. Proposing a novel combined cycle for optimal exergy recovery of liquefied natural gas. Heat Mass Transfer 48, 1309–1317 (2012). https://doi.org/10.1007/s00231-012-0977-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-012-0977-y