Abstract
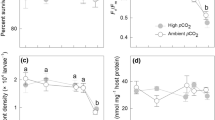
To evaluate the effects of temperature and pCO2 on coral larvae, brooded larvae of Pocillopora damicornis from Nanwan Bay, Taiwan (21°56.179′N, 120°44.85′E), were exposed to ambient (419–470 μatm) and high (604–742 μatm) pCO2 at ~25 and ~29 °C in two experiments conducted in March 2010 and March 2012. Larvae were sampled from four consecutive lunar days (LD) synchronized with spawning following the new moon, incubated in treatments for 24 h, and measured for respiration, maximum photochemical efficiency of PSII (F v/F m), and mortality. The most striking outcome was a strong effect of time (i.e., LD) on larvae performance: respiration was affected by an LD × temperature interaction in 2010 and 2012, as well as an LD × pCO2 × temperature interaction in 2012; F v/F m was affected by LD in 2010 (but not 2012); and mortality was affected by an LD × pCO2 interaction in 2010, and an LD × temperature interaction in 2012. There were no main effects of pCO2 in 2010, but in 2012, high pCO2 depressed metabolic rate and reduced mortality. Therefore, differences in larval performance depended on day of release and resulted in varying susceptibility to future predicted environmental conditions. These results underscore the importance of considering larval brood variation across days when designing experiments. Subtle differences in experimental outcomes between years suggest that transgenerational plasticity in combination with unique histories of exposure to physical conditions can modulate the response of brooded coral larvae to climate change and ocean acidification.



Similar content being viewed by others
References
Agostini S, Suzuki Y, Higuchi T, Casereto BE, Yoshinaga K, Nakano Y, Fujimura H (2012) Biological and chemical characteristics of the coral gastric cavity. Coral Reefs 31:147–156
Agrawal AA, LaForsch C, Tollrian R (1999) Transgenerational induction of defenses in animals and plants. Nature 401:60–63
Albright R (2011) Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J Mar Biol. doi:10.1155/2011/473615
Albright R, Langdon C (2011) Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob Change Biol 17:2478–2487
Albright R, Mason B, Langdon C (2008) Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 27:485–900
Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc Natl Acad Sci USA 107:20400–20404
Anlauf H, D’Croz L, O’Dea A (2011) A corrosive concoction: the combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J Exp Mar Biol Ecol 397:13–20
Baird AH, Gilmour JP, Kamiki TM, Nonaka M, Pratchett MS, Yamamoto H, Yamasaki H (2006) Temperature tolerance of symbiotic and non-symbiotic coral larvae. Proceedings of the 10th international coral reef symposium, pp 38–42
Ball EE, Hayward DC, Reece-Hoyes JS, Hislop NR, Samuel G, Saint R, Harrison PL, Miller DJ (2002) Coral development: from classical embryology to molecular control. Int J Dev Biol 46:671–678
Bassim KM, Sammarco PW (2003) Effects of temperature and ammonium on larval development and survivorship in a scleractinian coral (Diploria strigosa). Mar Biol 142:241–252
Bassim KM, Sammarco PW, Snell TL (2002) Effects of temperature on success of (self and non-self) fertilization and embryogenesis in Diploria strigosa (Cnidaria, Scleractinia). Mar Biol 140:479–488
Bhagooli R, Hidaka M (2003) Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. J Exp Mar Biol Ecol 291:181–197
Black KP (1993) The relative importance of local retention and inter-reef dispersal of neutrally buoyant material on coral reefs. Coral Reefs 12:43–53
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Chua MC, Leggat B, Moya A, Baird AH (in press) Near-future reductions in pH will have no consistent ecological effects on the early life history stages of reef corals. Mar Ecol Prog Ser. doi:10.3354/meps10318
Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst 10:Q07005. doi:10.1029/2009GC002411
Cowan RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1:443–466
Cumbo VR, Fan TY, Edmunds PJ (2012a) Effects of exposure duration on the response of Pocillopora damicornis larvae to elevated temperature and high pCO2. J Exp Mar Biol Ecol 439:100–107
Cumbo VR, Fan TY, Edmunds PJ (2012b) Physiological development of brooded larvae from two pocilloporid corals in Taiwan. Mar Biol. doi:10.1007/s00227-012-2046-y
de Putron SJ, McCorkle DC, Cohen AL, Dillon AB (2011) The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs 30:321–328
Detlef PV, Edmonds J, Kainuma M, Riahi K, Thomason A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F, Masui T, Meinshausen M, Nakicenovic N, Smith SJ, Rose SK (2011) The representative concentration pathways: an overview. Clim Change 109:5–31
Dickson AG, Sabine CL, Christian JR (eds) (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3, p 191
Dufault AM, Cumbo VR, Fan TY, Edmunds PJ (2012) Effects of diurnally oscillating pCO2 on calcification and survival of coral recruits. Proc R Soc Lond B. doi:10.1098/rspb.2011.2545
Edmunds PJ, Davies PS (1988) Post-illumination stimulation of the respiration rate in the coral Porites porites. Coral Reefs 7:7–9
Edmunds PJ, Gates RD, Gleason DF (2001) The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Mar Biol 139:981–989
Edmunds PJ, Gates RD, Leggat W, Hoegh-Guldberg O, Allen-Requa L (2005) The effect of temperature on the size and population density of dinoflagellates in larvae on the reef coral Porites astreoides. Invert Biol 124:185–193
Edmunds PJ, Cumbo VR, Fan TY (2011) Effects of temperature on the respiration of brooded larvae from tropical reef corals. J Exp Biol 214:2783–2790
Enriquez S, Mendez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50:1025–1032
Fan TY, Li JJ, Ie SX, Fang LS (2002) Lunar periodicity of larval release by pocilloporid corals in southern Taiwan. Zool Stud 41:288–294
Fan TY, Lin KH, Kuo FW, Soong K, Liu LL, Fang LS (2006) Diel patterns of larval release by five brooding scleractinian corals. Mar Ecol Prog Ser 321:133–142
Fangue NA, O’Donnell MJ, Sewell MA, Matson PG, MacPherson AC, Hoffman GE (2010) A laboratory-based, experimental system for the study of ocean acidification effects on marine invertebrate larvae. Limnol Oceanogr Methods 8:441–452
Gaither MR, Rowan R (2010) Zooxanthellar symbiosis in planula larvae of the coral Pocillopora damicornis. J Exp Mar Biol Ecol 386:45–53
Garcia HE, Gordon LI (1992) Solubility in seawater: better fitting solutions. Limnol Oceanogr 37:1307–1312
Gleason DF, Hofmann DK (2011) Coral larvae: from gametes to recruits. J Exp Mar Biol Ecol 408:42–57
Gleason DF, Edmunds PJ, Gates RD (2006) Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar Biol 148:503–512
Gleason DF, Danilowicz BS, Nolan CJ (2009) Reef waters stimulate substratum exploration in planulae from brooding Caribbean corals. Coral Reefs 28:549–554
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 46:265–282
Hadfield MG (2000) Why and how marine-invertebrate larvae metamorphose so fast. Cell Dev Biol 11:437–443
Hamdoun A, Epel D (2007) Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA 104:1745–1750
Harland AD, Davies PS (1995) Symbiont photosynthesis increases both respiration and photosynthesis in the symbiotic sea anemone Anemonia viridis. Mar Biol 123:715–722
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherlands, pp 59–85
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Ecosystems of the world, vol 25., Coral reefsElsevier, Amsterdam, pp 133–207
Herndl GJ, Velimirov B (1985) Bacteria in the coelenterons of Anthozoa: control of coelenteric bacterial density by the coelenteric fluid. J Exp Mar Biol Ecol 93:115–130
Heyward AJ, Negri AP (2010) Plasticity of larval pre-competency in response to temperature: observations on multiple broadcast spawning coral species. Coral Reefs 29:631–636
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Fresh Res 50:839–866
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthuga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hofmann GE, Barry JP, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA (2010) The effect of ocean acidification on calcifying organisms in marine ecosystems: an organism-to-ecosystem perspective. Annu Rev Evol Syst 41:127–147
IPCC (2007) Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Reisinger A (eds)]. IPCC, Geneva, Switzerland, 104 pp
Isomura N, Nishihara M (2001) Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20:309–315
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant, Cell Environ 21:1219–1230
Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, Opdyke BN (1999) Goechemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434
Lee HJ, Chao SY, Fan KL, Kuo TY (1999) Tide-induced eddies and upwelling in a semi-enclosed basin: Nan Wan. Estuar Coast Shelf Sci 49:775–787
Levitan DR (1996) Predicting optimal and unique egg sizes in free-spawning marine invertebrates. Am Nat 148:174–188
Markey KL, Baird AH, Humphrey C, Negri AP (2007) Insecticides and fungicide affect multiple coral life stages. Mar Ecol Prog Ser 330:127–137
Marlow HQ, Martindale MQ (2007) Embryonic development in two species of scleractinian coral embryos: Symbiodinium localization and mode of gastrulation. Evol Dev 9:355–367
Marshall DJ (2008) Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89:418–427
Marshall DJ, Keough MJ (2003) Variation in the dispersal potential of non-feeding invertebrate larvae: the desperate larva hypothesis and larval size. Mar Ecol Prog Ser 255:145–153
Marubini F, Ferrier-Pagès C, Furla P, Allemand D (2008) Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs 27:491–499
Mason B, Beard M, Miller MW (2011) Coral larvae settle at a higher frequency on red surfaces. Coral Reefs. doi:10.1007/s00338-011-0739-1
Morita M, Suwa R, Iguchi A, Nakamura M, Shimada K, Sakai K, Suzuki A (2009) Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18:103–107
Moya A, Huisman L, Ball EE, Hayward DC, Grasso LC, Chua CM, Woo HN, Gattuso JP, Foret S, Miller DJ (2012) Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol Ecol 21:2440–2454
Mundy CN, Babcock RC (1998) Role of light intensity and spectral quality in coral settlement: implications for depth-dependent settlement? Mar Ecol Prog Ser 223:235–255
Nakamura M, Ohki S, Suzuki A, Sakai K (2011) Coral larvae under ocean acidification: survival, metabolism and metamorphosis. PLoS ONE 6:e14521
Nakamura M, Morita M, Kurihara H, Mitarai S (2012) Expression of hsp70, hsp90 and hsf1 in the reef coral Acropora digitifera under prospective acidified conditions over the next several decades. Biol Open 1:75–81
Negri AP, Smity LD, Webster NS, Heyward AJ (2002) Understanding ship-grounding impacts on a coral reef: potential effects of anti-foulant paint contamination on coral recruitment. Mar Pollut Bull 44:111–117
Negri AP, Marshall PA, Heyward AJ (2007) Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs 26:759–763
Nozawa Y, Harrison PL (2007) Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis. Mar Biol 152:1181–1185
Nozawa Y, Okubo N (2011) Survival dynamics of reef coral larvae with special consideration of larval size and the genus Acropora. Biol Bull 220:15–22
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA 104:1266–1271
Orr JC (2011) Recent and future changes in ocean carbonate chemistry. In: Gattuso J-P, Hansonn L (eds) Ocean acidification. Oxford University Press, Oxford, pp 41–66
Permata WD, Kinzie RA, Hidakal M (2000) Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar Ecol Prog Ser 200:191–200
Pierrot D, Lewis E, Wallace DWR (2006) MS excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee
Putnam HM, Edmunds PJ, Fan TY (2008) Effects of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol Bull 215:135–142
Putnam HM, Edmunds PJ, Fan TY (2010) Effect of a fluctuating thermal regime on adult and larval reef corals. Invert Biol 129:199–209
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Raimondi PT, Morse ANC (2000) The consequence of complex larval behavior in a coral. Ecology 81:3193–3211
Randall CJ, Szmant AM (2009) Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 28:537–545
Rivest E, Hofmann G (2012) Metabolic plasticity in coral larvae under ocean acidification and warming. Proceedings of the 12th international coral reef symposium (abstracts) 158
Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O (2009) Early molecular responses of coral larvae to hyperthermal stress. Mol Ecol 18:1501–5114
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng TH, Kozyr A, Ono T, Rios AF (2004) The ocean sink for anthropogenic CO2. Science 305:367–371
Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408:850–853
Salinas S, Munch SB (2012) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15:159–163
Silverman J, Lazar B, Cao L, Caldeira K, Erez J (2009) Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys Res Lett 36:L05606
Stambler N (2011) Zooxanthellae: the yellow symbionts inside animals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherlands, pp 87–106
Stoddart JA, Black R (1985) Cycles of gametogenesis and planulation in the coral Pocillopora damicornis. Mar Ecol Prog Ser 23:153–164
Strathmann RR (1985) Feeding and nonfeeding larval development and life-history evolution in marine invertebrates. Annu Rev Ecol Syst 16:339–361
Suwa R, Nakamur M, Morita M, Shimada K, Iguchi A, Sakai K, Suzuki A (2010) Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish Sci 76:93–99
Webster NS, Uthicke S, Botte ES, Flores F, Negri A (2012) Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob Change Biol. doi:10.1111/gcb.12008
Yakovleva IM, Baird AH, Yamamoto HH, Bhagooli R, Nonaka M, Hidaka M (2009) Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar Ecol Prog Ser 378:105–112
Yeoh S-R, Dai C-F (2010) The reproduction of sexual and asexual larvae within single broods of the scleractinian coral, Pocillopora damicornis. Mar Biol 157:351–359
Zar JH (2010) Biostatistical analysis. Prentice Hall, Englewood Cliffs, NJ
Acknowledgments
This research was funded through the US National Science Foundation (BIO-OCE 08-44785) and contributes to the collaboration supported under the Memorandum of Understanding between California State University, Northridge, USA and National Dong Hwa University, Hualien, Taiwan. We thank A. Dufault, H.M. Putnam, S. Zamudio, A. Mayfield, P.J. Liu, Y.H Chen and O. Chan for field and laboratory assistance. Comments from four anonymous reviewers and H. Pörtner improved an earlier draft of this paper. This is contribution number 194 of the CSUN Marine Biology Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cumbo, V.R., Edmunds, P.J., Wall, C.B. et al. Brooded coral larvae differ in their response to high temperature and elevated pCO2 depending on the day of release. Mar Biol 160, 2903–2917 (2013). https://doi.org/10.1007/s00227-013-2280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2280-y