Abstract
DNA ligases are essential enzymes in all cells and have been proposed as targets for novel antibiotics. Efficient DNA ligase activity assays are thus required for applications in biomedical research. Here we present an enzyme-linked electrochemical assay based on two terminally tagged probes forming a nicked junction upon hybridization with a template DNA. Nicked DNA bearing a 5' biotin tag is immobilized on the surface of streptavidin-coated magnetic beads, and ligated product is detected via a 3' digoxigenin tag recognized by monoclonal antibody-alkaline phosphatase conjugate. Enzymatic conversion of napht-1-yl phosphate to napht-1-ol enables sensitive detection of the voltammetric signal on a pyrolytic graphite electrode. The technique was tested under optimal conditions and various situations limiting or precluding the ligation reaction (such as DNA substrates lacking 5′-phosphate or containing a base mismatch at the nick junction, or application of incompatible cofactor), and utilized for the analysis of the nick-joining activity of a range of recombinant Escherichia coli DNA ligase constructs. The novel technique provides a fast, versatile, specific, and sensitive electrochemical assay of DNA ligase activity.

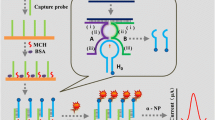
Enzyme-linked electrochemical detection of a ligated DNA strand using magnetic beads. Anti-digoxigenin antibody conjugate with alkaline phosphatase (ALP) is bound to digoxigenin label of the ligated product immobilized at streptavidin-coated magnetic beads via biotin tag on its opposite end. Then substrate for ALP (napht-1-yl phosphate) is added and enzymatically converted to napht-1-ol, an electroactive indicator, which is subsequently detected electrochemically at a carbon electrode





Similar content being viewed by others
References
Pascal JM (2008) DNA and RNA ligases: structural variations and shared mechanisms. Curr Opin Struct Biol 18:96–105
Tomkinson AE, Vijayakumar S, Pascal JM, Ellenberger T (2006) DNA ligases: structure, reaction mechanism, and function. Chem Rev 106:687–699
Wilkinson A, Day J, Bowater R (2001) Bacterial DNA ligases. Mol Microbiol 40:1241–1248
Shuman S (2009) DNA ligases: progress and prospects. J Biol Chem 284:17365–17369
Dwivedi N, Dube D, Pandey J, Singh B, Kukshal V, Ramachandran R, Tripathi RP (2008) NAD(+)-dependent DNA ligase: a novel target waiting for the right inhibitor. Med Res Rev 28:545–568
Korycka-Machala M, Rychta E, Brzostek A, Sayer HR, Rumijowska-Galewicz A, Bowater RP, Dziadek J (2007) Evaluation of NAD(+)-dependent DNA ligase of mycobacteria as a potential target for antibiotics. Antimicrob Agents Chemother 51:2888–2897
Gu WX, Wang TS, Maltais F, Ledford B, Kennedy J, Wei YY, Gross CH, Parsons J, Duncan L, Arends SJR, Moody C, Perola E, Green J, Charifson PS (2012) Design, synthesis, and biological evaluation of potent NAD(+)-dependent DNA ligase inhibitors as potential antibacterial agents. Part I: aminoalkoxypyrimidine carboxamides. Bioorg Med Chem Lett 22:3693–3698
Mills SD, Eakin AE, Buurman ET, Newman JV, Gao N, Huynh H, Johnson KD, Lahiri S, Shapiro AB, Walkup GK, Yang W, Stokes SS (2011) Novel bacterial NAD(+)-dependent DNA ligase inhibitors with broad-spectrum activity and antibacterial efficacy in vivo. Antimicrob Agents Chemother 55:1088–1096
Podos SD, Thanassi JA, Pucci MJ (2012) Mechanistic assessment of DNA ligase as an antibacterial target in Staphylococcus aureus. Antimicrob Agents Chemother 56:4095–4102
Surivet JP, Lange R, Hubschwerlen C, Keck W, Specklin JL, Ritz D, Bur D, Locher H, Seiler P, Strasser DS, Prade L, Kohl C, Schmitt C, Chapoux G, Ilhan E, Ekambaram N, Athanasiou A, Knezevic A, Sabato D, Chambovey A, Gaertner M, Enderlin M, Boehme M, Sippel V, Wyss P (2012) Structure-guided design, synthesis, and biological evaluation of novel DNA ligase inhibitors with in vitro and in vivo anti-staphylococcal activity. Bioorg Med Chem Lett 22:6705–6711
Wang TS, Duncan L, Gu WX, O'Dowd H, Wei YY, Perola E, Parsons J, Gross CH, Moody CS, Arends SJR, Charifson PS (2012) Design, synthesis, and biological evaluation of potent NAD(+)-dependent DNA ligase inhibitors as potential antibacterial agents. Part II: 4-amino-pyrido 2,3-d pyrimidin-5(8H)-ones. Bioorg Med Chem Lett 22:3699–3703
Wilkinson A, Smith A, Bullard D, Lavesa-Curto M, Sayer H, Bonner A, Hemmings A, Bowater R (2005) Analysis of ligation and DNA binding by Escherichia coli DNA ligase (LigA). Biochim Biophys Acta 1749:113–122
Baner J, Nilsson M, Isaksson A, Mendel-Hartvig M, Antson DO, Landegren U (2001) More keys to padlock probes: mechanisms for high-throughput nucleic acid analysis. Curr Opin Biotechnol 12:11–15
Shapiro AB, Eakin AE, Walkup GK, Rivin O (2011) A high-throughput fluorescence resonance energy transfer-based assay for DNA ligase. J Biomol Screen 16:486–493
Liu LF, Tang ZW, Wang KM, Tan WH, Li J, Guo QP, Meng XX, Ma CB (2005) Using molecular beacon to monitor activity of E-coli DNA ligase. Analyst 130:350–357
Scott BOS, Lavesa-Curto M, Bullard DR, Butt JN, Bowater RP (2006) Immobilized DNA hairpins for assay of sequential breaking and joining of DNA backbones. Anal Biochem 358:90–98
Luan Q, Xue Y, Yao X, Lu W (2010) Hairpin DNA probe based surface plasmon resonance biosensor used for the activity assay of E. coli DNA ligase. Analyst 135:414–418
He XX, Ni XQ, Wang YH, Wang KM, Jian LX (2011) Electrochemical detection of nicotinamide adenine dinucleotide based on molecular beacon-like DNA and E. coli DNA ligase. Talanta 83:937–942
Wu ZS, Jiang JH, Shen GL, Yu RQ (2007) Highly sensitive DNA detection and point mutation identification: an electrochemical approach based on the combined use of ligase and reverse molecular beacon. Hum Mutat 28:630–637
Pang LL, Li JS, Jiang JH, Le Y, Shen GL, Yu RQ (2007) A novel detection method for DNA point mutation using QCM based on Fe3O4/Au core/shell nanoparticle and DNA ligase reaction. Sensors Actuators B Chem 127:311–316
Zhang P, Chu X, Xu XM, Shen GL, Yu RQ (2008) Electrochemical detection of point mutation based on surface ligation reaction and biometallization. Biosens Bioelectron 23:1435–1441
Vacek J, Cahova K, Palecek E, Bullard DR, Lavesa-Curto M, Bowater RP, Fojta M (2008) Label-free electrochemical monitoring of DNA ligase activity. Anal Chem 80:7609–7613
Palecek E, Fojta M (2007) Magnetic beads as versatile tools for electrochemical DNA and protein biosensing. Talanta 74:276–290
Sambrook J, Russell DW (2001) Molecular cloning—a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, NY
Goffin C, Bailly V, Verly WG (1987) Nicks 3' or 5' to AP sites or to mispaired bases, and one-nucleotide gaps can be sealed by T4 DNA-ligase. Nucleic Acids Res 15:8755–8771
Tsiapali CM, Narang SA (1970) On fidelity of phage T4-induced polynucleotide ligase in joining of chemically synthesized deoxyribonucleotides. Biochem Biophys Res Commun 39:631–636
Kuramitz H (2009) Magnetic microbead-based electrochemical immunoassays. Anal Bioanal Chem 394:61–69
Palecek E, Bartosik M (2012) Electrochemistry of nucleic acids. Chem Rev 112:3427–3481
Acknowledgments
This work was supported by the Czech Science Foundation (grant P206/11/P739 to P.H. and P206/11/1638 to M.F.) and by the Grant Agency of the ASCR (grant IAA400040901), and by the Academy of Sciences of the Czech Republic (RVO 68081707).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stejskalová, E., Horáková, P., Vacek, J. et al. Enzyme-linked electrochemical DNA ligation assay using magnetic beads. Anal Bioanal Chem 406, 4129–4136 (2014). https://doi.org/10.1007/s00216-014-7811-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7811-y