Abstract
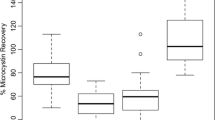
Tetrodotoxin (TTX) is a potent neurotoxin emerging in European waters due to increasing ocean temperatures. Its detection in seafood is currently performed as a consequence of using the Association of Analytical Communities (AOAC) mouse bioassay (MBA) for paralytic shellfish poisoning (PSP) toxins, but TTX is not monitored routinely in Europe. Due to ethical and performance-related issues associated with this bioassay, the European Commission has recently published directives extending procedures that may be used for official PSP control. An AOAC-accredited high-performance liquid chromatography (HPLC) method has now been accepted by the European Union as a first action screening method for PSP toxins to replace the MBA. However, this AOAC HPLC method is not capable of detecting TTX, so this potent toxin would be undetected; thereby, a separate method of analysis is required. Surface plasmon resonance (SPR) optical biosensor technology has been proven as a potential alternative screening method to detect PSP toxins in seafood. The addition of a similar SPR inhibition assay for TTX would complement the PSP assay in removing the MBA. The present report describes the development and single laboratory validation in accordance with AOAC and IUPAC guidelines of an SPR method to be used as a rapid screening tool to detect TTX in the sea snail Charonia lampas lampas, a species which has been implicated in 2008 in the first case of human TTX poisoning in Europe. As no current regulatory limits are set for TTX in Europe, single laboratory validation was undertaken using those for PSP toxins at 800 μg/kg. The decision limit (CCα) was 100 μg/kg, with the detection capability (CCβ) found to be ≤200 μg/kg. Repeatability and reproducibility were assessed at 200, 400, and 800 μg/kg and showed relative standard deviations of 8.3, 3.8, and 5.4 % and 7.8, 8.3, and 3.7 % for both parameters at each level, respectively. At these three respective levels, the recovery of the assay was 112, 98, and 99 %.



Similar content being viewed by others
References
Moczydlowski EG (2013) The molecular mystique of TTX. Toxicon 63:165–183
Yasumoto T, Yotsu-Yamashita M (1996) Chemical and etiological studies on TTX and its analogs. Toxin Rev 15(2):81–90
Fozzard HA, Lipkind GM (2010) The TTX binding site is within the outer vestibule of the sodium channel. Mar Drugs 8:219–234
Williams BL (2010) Behavioral and chemical ecology of marine organisms with respect to tetrodotoxin. Mar Drugs 8:381–398
French RJ, Yoshikami D, Sheets MF, Olivera BM (2010) The tetrodotoxin receptor of voltage-gated sodium channels—perspectives from interactions with μ-conotoxins. Mar Drugs 8:2153–2161
Lee HL, Ruben PC (2008) Interaction between voltage-gated sodium channels and the neurotoxin, TTX. Channels 2:407–412
Narahashi T (2001) Pharmacology of TTX. Toxin Rev 20:67–84
Narahashi T (2008) TTX: a brief history. Proc Jpn Acad Ser B Phys Biol Sci 84:147–154
Noguchi T, Arakawa O (2008) TTX—distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar Drugs 6:220–242
Saoudi M, Abdelmouleh A, El Feki A (2010) TTX: a potent marine toxin. Toxin Rev 29(2):60–70
Alcaraz A, Whipple RE, Gregg HR, Andresen BD, Grant PM (1999) Analysis of TTX. Forensic Sci Int 99(1):35–45
How CK, Chern CH, Huang YC, Wang LM, Lee CH (2003) TTX poisoning. Am J emerg med 21:51–54
Hungerford JM, Committee on Natural Toxins and Food Allergens (2006) Marine and freshwater toxins. J AOAC Int 89(1):248–269
Yakes BJ, Deeds J, White K, Degrasse SL (2011) Evaluation of surface plasmon resonance biosensors for detection of TTX in food matrices and comparison to analytical methods. J Agric Food Chem 59(3):839–846
Rodriguez P, Alfonso A, Vale C, Alfonso C, Vale P, Tellez A, Botana LM (2008) First toxicity report of TTX and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal Chem 80:5622–5629
Fernández-Ortega JF, Morales-de los Santos JM, Herrera-Gutiérrez ME, Fernández-Sánchez V, Rodríguez Loureo P, Alfonso Rancaño A, Téllez-Andrade A (2010) Seafood intoxication by TTX: first case in Europe. J Emerg Med 39:612–617
Bentur Y, Ashkar J, Lurie Y, Levy Y, Azzam ZS, Litmanovich M, Golik M, Gurevych B, Golani D, Eisenman A (2008) Lessepsian migration and TTX poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 52:964–968
Katikou P, Georgantelis D, Sinouris N, Petsi A, Fotaras T (2009) First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 54:50–55
Rodriguez P, Alfonso A, Otero P, Katikou P, Georgantelis D, Botana LM (2012) Liquid chromatography–mass spectrometry method to detect TTX and its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem 132(2):1103–1111
Silva M, Azevedo J, Rodriguez P, Alfonso A, Botana LM, Vasconcelos V (2012) New gastropod vectors and TTX potential expansion in temperate waters of the Atlantic Ocean. Mar drugs 10:712–726
Commission Regulation (EC) no. 854/2004 of the European Parliament and of the Council of 29 April (2004) Laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off J Eur Communities L226:83–126
AOAC International (2005) AOAC official method 959.08. In: Official methods of analysis of AOAC International, section 49.10.01, 18th ed. AOAC International, Gaithersburg
Yasumoto T (1991) The manual for methods of food sanitation tests. Japan Food Hygienic Association, Tokyo
AOAC International (2005) AOAC official method 2005.06. In: Official methods of analysis of AOAC International, section 49.10.03, 18th ed. AOAC International, Gaithersburg
Doucette GJ, Powell CL, Do EU, Byon CY, Cleves F, McClain SG (2000) Evaluation of 11-[H3]-TTX use in a heterologous receptor binding assay for PSP toxins. Toxicon 38:1465–1474
Chen CY, Chou HN (1998) Detection of TTX by high performance liquid chromatography in lined moon shell and puffer fish. Acta Zool Taiwanica 9(1):41–48
Kouassi Nzoughet J, Campbell K, Barnes P, Cooper KM, Chevallier OP, Elliott CT (2013) Comparison of sample preparation methods and validation of an UPLC-MS/MS procedure to EU guidelines for the quantification of TTX present in marine gastropods and pufferfish. Food Chem 136:1584–1589
Kawatsu K, Hamano Y, Yoda T, Teano Y, Shibata T (1997) Rapid and highly sensitive enzyme immunoassay for quantitative determination of TTX. Jpn J Med Sci Biol 50:133–150
Matsumura K, Fukiya S (1992) Indirect competitive enzyme immunoassay for TTX using a biotin avidin system. J AOAC Int 75(5):883–886
Raybould TJG, Bignami GS, Inouye LK, Simpson SB, Byrnes JB, Grothaus PG, Vann DC (1992) A monoclonal antibody based immunoassay for detecting TTX in biological samples. J Clin Lab Anal 6:65–72
Tao J, Wei WJ, Nan L, Lei LH, Hui HC, Fen GX, Jun LY, Jing Z, Rong J (2010) Development of competitive indirect ELISA for the detection of TTX and a survey of the distribution of TTX in the tissues of wild puffer fish in the waters of south-east China. Food Addit Contam 27(11):1589–1597
Taylor AD, Ladd J, Etheridge S, Deeds J, Hall S, Jiang S (2008) Quantitative detection of TTX (TTX) by a surface plasmon resonance (SPR) sensor. Sensors Actuators B 130:120–128
Vaisocherova H, Taylor AD, Jiang S, Hegnerova K, Vala M, Homola J, Yakes BJ, Deeds J, DeGrasse S (2011) Surface plasmon resonance biosensor for determination of TTX: prevalidation study. J AOAC Int 94:596–604
Traynor I, Plumpton L, Fodey TL, Higgins C, Elliott CT (2006) Immunobiosensor detection of domoic acid as a screening test in bivalve mollusks: comparison with LC-based analysis. J AOAC Int 89:868–872
Stewart LD, Hess P, Connolly L, Elliott CT (2009) Development and single-laboratory validation of a pseudofunctional biosensor immunoassay for the detection of the okadaic acid group of toxins. Anal Chem 81:10208–10214
Campbell K, Haughey SA, van den Top H, van Egmond H, Vilarino N, Botana LM, Elliott CT (2010) Single laboratory validation of a surface plasmon resonance biosensor screening method for paralytic shellfish poisoning toxins. Anal Chem 82:2977–2988
Van den Top H, Haughey S, Vilariño N, Botana L, Van Egmond H, Elliott CT, Campbell K (2011) Surface plasmon resonance biosensor screening method for paralytic shellfish poisoning toxins: a pilot interlaboratory study. Anal Chem 83:4206–4213
Campbell K, Stewart LD, Fodey TL, Haughey SA, Doucette GJ, Kawatsu K, Elliott CT (2007) An assessment of specific binding proteins suitable for the detection of paralytic shellfish poisons (PSP) using optical biosensor technology. Anal Chem 79(15):5906–5914
Bates HA, Kostriken R, Rapoport H (1978) A chemical assay for saxitoxin. Improvements and modifications. J Agric food chem 26:252–254
Thompson M, Ellison SLR, Wood R (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC technical report). Pure Applied Chemistry 74:835–855
AOAC guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals. Available at http://www.aoac.org/Official_Methods/slv_guidelines.pdf. Accessed 22 April 2013
European Commission Decision (EC) no. 2002/657/EC implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Communities L221:8–34
Acknowledgments
This research was partly funded through The Interreg Project “ATLANTOX: Advanced Tests about New Toxins appeared in the Atlantic Area” and the CONffIDENCE: EU FP7 project “CONtaminants in Food and Feed: Inexpensive Detection for Control of Exposure” financially supported by the European Commission (grant agreement number 211326—collaborative project). The authors would like to thank Panagiota Katikou of the National Reference Laboratory for Marine Biotoxins in Greece for the provision of puffer fish samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Rapid Detection in Food and Feed with guest editors Rudolf Krska and Michel Nielen.
Rights and permissions
About this article
Cite this article
Campbell, K., Barnes, P., Haughey, S.A. et al. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal Bioanal Chem 405, 7753–7763 (2013). https://doi.org/10.1007/s00216-013-7106-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7106-8