Abstract
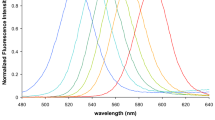
Biomarker assays may be useful for screening and diagnosis of cancer if a set of molecular markers can be quantified and statistically differentiated between cancerous cells and healthy cells. Markers of disease are often present at very low concentrations, so methods capable of low detection limits are required. Quantum dots (QDs) are nanoparticles that are emerging as promising probes for ultrasensitive detection of cancer biomarkers. QDs attached to antibodies, aptamers, oligonucleotides, or peptides can be used to target cancer markers. Their fluorescent properties have enabled QDs to be used as labels for in-vitro assays to quantify biomarkers, and they have been investigated as in-vivo imaging agents. QDs can be used as donors in assays involving fluorescence resonance energy transfer (FRET), or as acceptors in bioluminescence resonance energy transfer (BRET). The nanoparticles are also capable of electrochemical detection and are potentially useful for “lab-on-a-chip” applications. Recent developments in silicon QDs, non-blinking QDs, and QDs with reduced-size and controlled-valence further make these QDs bioanalytically attractive because of their low toxicity, biocompatibility, high quantum yields, and diverse surface modification flexibility. The potential of multiplexed sensing using QDs with different wavelengths of emission is promising for simultaneous detection of multiple biomarkers of disease.

Quantum dots have been conjugated to affinity probes to assay for cancer biomarkers including proteins, peptides, DNA, and whole cells





Similar content being viewed by others
Abbreviations
- AFP:
-
α-Fetoprotein
- BPDE:
-
Benzo[a]pyrene diol epoxide
- BRET:
-
Bioluminescence resonance energy transfer
- CA:
-
Cancer antigen
- CEA:
-
Carcinoembryonic antigen
- CK18:
-
Cytokeratin 18
- DNA:
-
Deoxyribonucleic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FRET:
-
Fluorescence resonance energy transfer
- HER2:
-
Human epidermal growth factor receptor 2
- IgG:
-
Immunoglobulin G
- MMP:
-
Matrix metalloproteinase
- MUC1:
-
Mucin 1
- PCR:
-
Polymerase chain reaction
- PEG:
-
Poly(ethylene glycol)
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- QD:
-
Quantum dot
- RCA:
-
Rolling circle amplification
- RNA:
-
Ribonucleic acid
- SELEX:
-
Systematic evolution of ligands by exponential enrichment
- uPA:
-
Urokinase-type plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225
Ullah MF, Aatif M (2009) The footprints of cancer development: cancer biomarkers. Cancer Treat Rev 35:193
Ludwig JA, Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5:845
Bast RC, Badgwell D, Lu Z et al (2005) New tumor markers: CA125 and beyond. Int J Gynecol Cancer 15:274
Smith R, Cokkinides V, Brauley OW (2008) Cancer screening in the United States, 2008: A review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin 58:161
Zhang H, Zhao Q, Li X et al (2007) Ultrasensitive assays for proteins. Analyst 132:724
Michalet X, Pinaud FF, Bentolila LA et al (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538
Medintz IL, Uyeda HT, Goldman ER et al (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435
Medintz IL, Mattoussi H, Clapp AR (2008) Potential clinical applications of quantum dots. Int J Nanomed 3:151
Frasco MF, Chaniotakis N (2010) Bioconjugated quantum dots as fluorescent probes for bioanalytical applications. Anal Bioanal Chem 396:229
Wilson WL, Szajowski PF, Brus LE (1993) Quantum confinement in size-selected. Surface-oxidized silicon nanocrystals. Science 262:1242
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933
Zhang CY, Yeh HC, Kuroki MT et al (2005) Single-quantum-dot-based DNA nanosensor. Nat Mater 4:826
Medintz IL, Clapp AR, Mattoussi H et al (2003) Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater 2:630
Bagalkot V, Zhang L, Levy-Nissenbaum E et al (2007) Quantum dot - Aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on Bi-fluorescence resonance energy transfer. Nano Lett 7:3065
Cheng AKH, Su H, Wang YA et al (2009) Aptamer-based detection of epithelial tumor marker mucin 1 with quantum dot-based fluorescence readout. Anal Chem 81:6130
Boeneman K, Mei BC, Dennis AM et al (2009) Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J Am Chem Soc 131:3828
Dabbousi BO, RodriguezViejo J, Mikulec FV et al (1997) (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B 101:9463
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S et al (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Meth 5:763
Bruchez M, Moronne M, Gin P et al (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013
Chan WCW, Nie SM (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016
Jaiswal JK, Mattoussi H, Mauro JM et al (2003) Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol 21:47
Hanaki K, Momo A, Oku T et al (2003) Semiconductor quantum dot/albumin complex is a long-life and highly photostable endosome marker. Biochem Biophys Res Commun 302:496
Goldman ER, Clapp AR, Anderson GP et al (2004) Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal Chem 76:684
Erogbogbo F, Yong K, Roy I et al (2008) Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2:873
Warner JH, Hoshino A, Yamamoto K et al (2005) Water-soluble photoluminescent silicon quantum dots. Angew Chem Intl Ed 44:4550
He Y, Su Y, Yang X et al (2009) Photo and pH stable. Highly-luminescent silicon nanospheres and their bioconjugates for immunofluorescent cell imaging. J Am Chem Soc 131:4434
Zhang L, Shen X, Liang H et al (2010) Hot-injection synthesis of highly luminescent and monodisperse CdS nanocrystals using thioacetamide and cadmium source with proper reactivity. J Colloid Interface Sci 342:236
Talapin DV, Mekis I, Gotzinger S et al (2004) CdSe/CdS/ZnS and CdSe/ZnSe/ZnS core-shell-shell nanocrystals. J Phys Chem B 108:18826
Kucur E, Boldt FM, Cavaliere-Jaricot S et al (2007) Quantitative analysis of cadmium selenide nanocrystal concentration by comparative techniques. Anal Chem 79:8987
Deng Z, Schulz O, Lin S et al (2010) Aqueous synthesis of zinc blende CdTe/CdS magic-core/thick-shell tetrahedral-shaped nanocrystals with emission tunable to near-infrared. J Am Chem Soc 132:5592
Smith AM, Mohs AM, Nie S (2009) Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat Nanotechnol 4:56
Zhang W, Chen G, Wang J et al (2009) Design and synthesis of highly luminescent near-infrared-emitting water-soluble CdTe/CdSe/ZnS core/shell/shell quantum dots. Inorg Chem 48:9723
Xu S, Kumar S, Nann T (2006) Rapid synthesis of high-quality InP nanocrystals. J Am Chem Soc 128:1054
Xu S, Ziegler J, Nann T (2008) Rapid synthesis of highly luminescent InP and InP/ZnS nanocrystals. J Mat Chem 18:2653
Xie R, Peng X (2009) Synthesis of Cu-doped InP nanocrystals (d-dots) with ZnSe diffusion barrier as efficient and color-tunable NIR emitters. J Am Chem Soc 131:10645
Fernee MJ, Thomsen E, Jensen P et al (2006) Highly efficient luminescence from a hybrid state found in strongly quantum confined PbS nanocrystals. Nanotechnology 17:956
Hinds S, Myrskog S, Levina L et al (2007) NIR-emitting colloidal quantum dots having 26% luminescence quantum yield in buffer solution. J Am Chem Soc 129:7218
Du H, Chen CL, Krishnan R et al (2002) Optical properties of colloidal PbSe nanocrystals. Nano Lett 2:1321
Hansen JA, Wang J, Kawde A et al (2006) Quantum-dot/aptamer-based ultrasensitive multi-analyte electrochemical biosensor. J Am Chem Soc 128:2228
Liu G, Wang J, Kim J et al (2004) Electrochemical coding for multiplexed immunoassays of proteins. Anal Chem 76:7126
Wang J, Liu G, Wu H et al (2008) Quantum-dot-based electrochemical immunoassay for high-throughput screening of the prostate-specific antigen. Small 4:82
Wu XY, Liu HJ, Liu JQ et al (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 21:41
Gao XH, Cui YY, Levenson RM et al (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 22:969
Dubertret B, Skourides P, Norris DJ et al (2002) In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298:1759
Gerion D, Pinaud F, Williams SC et al (2001) Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J Phys Chem B 105:8861
Jokerst JV, Raamanathan A, Christodoulides N et al (2009) Nano-bio-chips for high performance multiplexed protein detection: Determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bioelectron 24:3622
Hamula CLA, Guthrie JW, Zhang H et al (2006) Selection and analytical applications of aptamers. Trends Anal Chem 25:681
Daniels DA, Chen H, Hicke BJ et al (2003) A tenascin-C aptamer identified by tumor cell SELEX: Systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci U S A 100:15416
Shangguan D, Li Y, Tang Z et al (2006) Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci U S A 103:11838
Algar WR, Krull UJ (2009) Toward a multiplexed solid-phase nucleic acid hybridization assay using quantum dots as donors in fluorescence resonance energy transfer. Anal Chem 81:4113
Sapsford KE, Farrell D, Sun S et al (2009) Monitoring of enzymatic proteolysis on a electroluminescent-CCD microchip platform using quantum dot-peptide substrates. Sens Actuators B-Chem 139:13
Shi L, De Paoli V, Rosenzweig N et al (2006) Synthesis and application of quantum dots FRET-based protease sensors. J Am Chem Soc 128:10378
Hu FQ, Ran YL, Zhou ZA et al (2006) Preparation of bioconjugates of CdTe nanocrystals for cancer marker detection. Nanotechnology 17:2972
Chu TC, Shieh F, Lavery LA et al (2006) Labeling tumor cells with fluorescent nanocrystal-aptamer bioconjugates. Biosens Bioelectron 21:1859
Ghazani AA, Lee JA, Klostranec J et al (2006) High throughput quantification of protein expression of cancer antigens in tissue microarray using quantum dot nanocrystals. Nano Lett 6:2881
Chen X, Deng Y, Lin Y et al (2008) Quantum dot-labeled aptamer nanoprobes specifically targeting glioma cells. Nanotechnology 19:235105
Hirata E, Arakawa Y, Shirahata M et al (2009) Endogenous tenascin-C enhances glioblastoma invasion with reactive change of surrounding brain tissue. Cancer Sci 100:1451
Weng KC, Noble CO, Papahadjopoulos-Sternberg B et al (2008) Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett 8:2851
Smith BR, Cheng Z, De A et al (2008) Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett 8:2599
Choi HS, Liu W, Liu F et al (2010) Design considerations for tumour-targeted nanoparticles. Nat Nano 5:42
Ntziachristos V, Bremer C, Weissleder R (2003) Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol 13:195
Smith AM, Mancini MC, Nie S (2009) Bioimaging: second window for in vivo imaging. Nat Nano 4:710
Xiao Y, Gao X, Gannot G et al (2008) Quantitation of HER2 and telomerase biomarkers in solid tumors with IgY antibodies and nanocrystal detection. Int J Cancer 122:2178
Sweeney E, Ward TH, Gray N et al (2008) Quantitative multiplexed quantum dot immunohistochemistry. Biochem Biophys Res Commun 374:181
Kerman K, Endo T, Tsukamoto M et al (2007) Quantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopy. Talanta 71:1494
Wang Z, Lu M, Wang X et al (2009) Quantum dots enhanced ultrasensitive detection of DNA adducts. Anal Chem 81:10285
Randerath K, Reddy MV, Gupta RC (1981) P32-labeling test for DNA damage. Proc Natl Acad Sci U S A 78:6126
Sancar A (1994) Mechanisms of Dna excision-repair. Science 266:1954
Doll R, Peto R (1981) The causes of cancer - quantitative estimates of avoidable risks of cancer in the United-States today. J Natl Cancer Inst 66:1191
Cheng W, Yan F, Ding L et al (2010) Cascade signal amplification strategy for subattomolar protein detection by rolling circle amplification and quantum dots tagging. Anal Chem 82:3337
Zhang H, Wang Z, Li X et al (2006) Ultrasensitive detection of proteins by amplification of affinity aptamers. Angew Chem Intl Ed 45:1576
Algar WR, Krull UJ (2008) Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal Bioanal Chem 391:1609
Medintz IL, Mattoussi H (2009) Quantum dot-based resonance energy transfer and its growing application in biology. Phys Chem Chem Phys 11:17
Clapp AR, Medintz IL, Mattoussi H (2006) Forster resonance energy transfer investigations using quantum-dot fluorophores. Chemphyschem 7:47
Clapp AR, Medintz IL, Mauro JM et al (2004) Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J Am Chem Soc 126:301
Algar WR, Krull UJ (2010) Multiplexed interfacial transduction of nucleic acid hybridization using a single color of immobilized quantum dot donor and two acceptors in fluorescence resonance energy transfer. Anal Chem 82:400
Brena RM, Huang TH, Plass C (2006) Quantitative assessment of DNA methylation: potential applications for disease diagnosis, classification, and prognosis in clinical settings. J Mol Med 84:365
Jones PA, Laird PW (1999) Cancer epigenetics comes of age. Nat Genet 21:163
Bailey VJ, Easwaran H, Zhang Y et al (2009) MS-qFRET: a quantum dot-based method for analysis of DNA methylation. Genome Res 19:1455
Breast Cancer Antigen CA15-3 Enzyme Immunoassay Test Kit (2009) MP Biomedicals, Orangeburg, NY. Accessed Dec 2 2009. http://www.mpbio.com/includes/technical/CA15-3ELISA,07BC1015.pdf
Medintz IL, Clapp AR, Brunel FM et al (2006) Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat Mater 5:581
Chang E, Miller JS, Sun JT et al (2005) Protease-activated quantum dot probes. Biochem Biophys Res Commun 334:1317
Kim Y, Oh Y, Oh E et al (2008) Energy transfer-based multiplexed assay of proteases by using gold nanoparticle and quantum dot conjugates on a surface. Anal Chem 80:4634
McCawley LJ, Matrisian LM (2000) Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 6:149
Devarajan E, Sahin AA, Chen JS et al (2002) Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21:8843
Xu Y, Piston DW, Johnson CH (1999) A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci U S A 96:151
Xia Z, Rao J (2009) Biosensing and imaging based on bioluminescence resonance energy transfer. Curr Opin Biotechnol 20:37
Xia Z, Xing Y, So M et al (2008) Multiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugation. Anal Chem 80:8649
Zajac A, Song D, Qian W et al (2007) Protein microarrays and quantum dot probes for early cancer detection. Colloid Surf B-Biointerfaces 58:309
Hu M, Yan J, He Y et al (2010) Ultrasensitive, multiplexed detection of cancer biomarkers directly in serum by using a quantum dot-based microfluidic protein chip. ACS Nano 4:488
Daniels R (2010) Delmar’s guide to laboratory and diagnostic tests. Delmar/Cengage Learning, Clifton Park
Wang J, Liu GD, Polsky R et al (2002) Electrochemical stripping detection of DNA hybridization based on cadmium sulfide nanoparticle tags. Electrochem Commun 4:722
Numnuam A, Chumbimuni-Torres KY, Xiang Y et al (2008) Aptamer-based potentiometric measurements of proteins using ion-selective microelectrodes. Anal Chem 80:707
Ho J-A, Lin Y, Wang L et al (2009) Carbon nanoparticle-enhanced immunoelectrochemical detection for protein tumor marker with cadmium sulfide biotracers. Anal Chem 81:1340
Cheng W, Ding L, Ding S et al (2009) A simple electrochemical cytosensor array for dynamic analysis of carcinoma cell surface glycans. Angew Chem Intl Ed 48:6465
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109:1948
Derfus AM, Chan WCW, Bhatia SN (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4:11
Hardman R (2006) A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 114:165
Rzigalinski BA, Strobl JS (2009) Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol 238:280
Shiohara A, Hanada S, Prabakar S et al (2010) Chemical reactions on surface molecules attached to silicon quantum dots. J Am Chem Soc 132:248
Wang X, Ren X, Kahen K et al (2009) Non-blinking semiconductor nanocrystals. Nature 459:686
Smith AM, Nie S (2009) Next-generation quantum dots. Nat Biotech 27:732
Howarth M, Liu W, Puthenveetil S et al (2008) Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat Meth 5:397
Park J, Gu L, von Maltzahn G et al (2009) Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater 8:331
Choi HS, Liu W, Misra P et al (2007) Renal clearance of quantum dots. Nat Biotechnol 25:1165
Algar WR, Krull UJ (2009) Developing mixed films of immobilized oligonucleotides and quantum dots for the multiplexed detection of nucleic acid hybridization using a combination of fluorescence resonance energy transfer and direct excitation of fluorescence. Langmuir 26:6041
Medintz IL, Farrell D, Susumu K et al (2009) Multiplex charge-transfer interactions between quantum dots and peptide-bridged ruthenium complexes. Anal Chem 81:4831
Medintz IL, Pons T, Trammell SA et al (2008) Interactions between redox complexes and semiconductor quantum dots coupled via a peptide bridge. J Am Chem Soc 130:16745
Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council of Canada, Canadian Institutes of Health Research, the Canada Research Chairs program, and Alberta Health and Wellness for their support. An Alberta Ingenuity Nanotechnology Scholarship (to MKW) and a China Scholarship Council visiting studentship (to JL) are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, M.K., Li, F., Li, J. et al. Use of quantum dots in the development of assays for cancer biomarkers. Anal Bioanal Chem 397, 3213–3224 (2010). https://doi.org/10.1007/s00216-010-3847-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3847-9