Abstract
Catalytic adsorptive stripping voltammetry (CAdSV) has been demonstrated at a bismuth film electrode (BiFE) in an injection-moulded electrochemical micro-flow cell. The polystyrene three-electrode flow cell was fabricated with electrodes moulded from a conducting grade of polystyrene containing 40% carbon fibre, one of which was precoated with Ag to enable its use as an on-chip Ag/AgCl reference electrode. CAdSV of Co(II) and Ni(II) in the presence of dimethylglyoxime (DMG) with nitrite employed as the catalyst was performed in order to assess the performance of the flow cell with an in-line plated BiFE. The injection-moulded electrodes were found to be suitable substrates for the formation of BiFEs. Key parameters such as the plating solution matrix, plating flow rate, analysis flow rate, solution composition and square-wave parameters have been characterised and optimal conditions selected for successful and rapid analysis of Co(II) and Ni(II) at the ppb level. The analytical response was linear over the range 1 to 20 ppb and deoxygenation of the sample solution was not required. The successful coupling of a microfluidic flow cell with a BiFE, thereby forming a “mercury-free” AdSV flow analysis sensor, shows promise for industrial and in-the-field applications where inexpensive, compact, and robust instrumentation capable of low-volume analysis is required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury electrodes, normally in the form of mercury films for flow systems, have been widely used for performing stripping voltammetry as a result of their high sensitivity and reproducibility, but, due to increasing concern and legislation regarding the toxicity of mercury, alternative working electrode (WE) materials have been investigated. Metals such as Au [1, 2], Ag [2, 3], Ir [4] and even W [5] as well as bare and coated carbon electrodes [6, 7] have all been investigated for stripping analysis of metals.
In 2000, bismuth-film electrodes [8] were mooted as a replacement for mercury-film electrodes, as bismuth exhibits negligible toxicity and is more environmentally friendly in comparison. Mercury use is increasingly regulated, motivating the search for mercury-free methods. For example, Sweden banned the sale of switches, apparatuses and instruments containing mercury in 1993 (Ordinance SFS 1991:1290) and instructed the Swedish Chemicals Inspectorate (KemI) to look into a total ban on the handling of mercury in Sweden, including chemicals used for analysis. The Inspectorate proposed that mercury for chemical analysis should be exempted from the general ban until 31st December 2008 in order to enable the development and implementation of mercury-free analysis methods [9]. In 2005 the EU commission proposed a ban on mercury exports by 2011 as part of a strategy to reduce mercury emissions and protect against exposure [10], and recently MEPs voted to bring this date forward to 2010 [11].
A variety of substrates have been used for bismuth films. While platinum [12–14] and gold [13] have both been investigated, the majority of films have been deposited on carbon substrates. Most studies have used glassy carbon [8, 13, 15–32], though wax-impregnated graphite [22, 24], pencil-lead graphite [33], carbon paste [13, 34–36], screen-printed carbon inks [21, 37, 38], carbon fibres [8, 13, 14] and boron-doped diamond [39] have all been employed as substrates. The use of Bi2O3 containing pastes [34, 40] and a bismuth bulk electrode [41] have also been reported.
The use of BiFE has recently been reviewed [42]. Bismuth-film electrodes have mainly been applied to anodic stripping voltammetry (ASV) and adsorptive stripping voltammetry (AdSV) techniques for metal ion analysis, although cathodic detection of nitrophenols [17, 27, 41] and amperometric detection of glucose [12] have also been reported. Cd [28], Pb [18, 37], Zn [31, 32] and mixtures of all three ions [8, 20, 22, 23, 33, 36, 41] have been the most extensively studied by ASV. Tl, Cu [16], In [16, 43], Mn [39] and Sn [44] have also been analysed. Fewer reports have been made employing AdSV, though Ni [15] and Co [28–30] and Ni with Co [24, 26] have been examined using dimethylglyoxime (DMG). Cr [21, 25, 45], U [46, 47], Al [48], V [49] and Mo [50] analysis by AdSV on bismuth-film electrodes has also been performed.
Stirred solutions have normally been used, though Economou et al. have reported the use of a rotating disc electrode [22–25] and a Nafion-coated BiFE in a thin layer flow cell (0.5 mm spacer) for sequential injection-ASV of metals [51, 52]. Hutton et al. generated an in-situ BiFE in a thin layer (0.3 mm spacer) flow cell (Bioanalytical Systems Inc., West Lafayette, IN, USA) for flow injection analysis of nitrophenols with cathodic amperometric detection [27].
Adsorptive stripping voltammetry of DMG-complexed Co and Ni has found widespread use in trace metal analysis. The enhancement of the Co-DMG response using nitrite, i.e. catalytic adsorptive stripping voltammetry (CAdSV), has also been the subject of several publications, for example van den Berg et al. [53, 54] and the review of catalytic systems by Bobrowski and Zarębski [55]. CAdSV of Co on a BiFE was reported by Krolicka et al. [29, 30] using an ex situ plated film on glassy carbon. Most BiFEs have been plated from acetic buffers, although Krolicka et al. used a plating solution of Bi(NO3)3 (0.02 M), LiBr (0.5 M), and HCl (1 M) with a plating potential of −0.25 V for 45 s [addition of bromide and strong acid enabled a highly concentrated Bi(III) plating solution to be produced]. Stripping was typically carried out in 0.1 M ammonia buffer (pH 9.2) containing DMG (1 × 10−4 M) and NaNO2 (0.5 M). Voltammograms were recorded in the differential pulse mode and a 15-fold enhancement of the Co-DMG stripping signal was reported.
Recently the authors reported the use of 40% carbon fibre-filled nylon 6/6 injection-moulded electrodes as working electrodes for the determination of Cu(II) by CV and ASV techniques [56].
In this work, the electrodes were moulded from a 40% carbon fibre-filled polystyrene, which was also found to be suitable for performing electrochemical detection. This paper presents the first report of the use of CAdSV at an in-line plated BiFE in an integrated three-electrode injection-moulded plastic micro flow cell.
Experimental
Instrumentation
Voltammetric measurements were performed using an Autolab electrochemical system (PSTAT 10, Eco Chemie B.V., Utrecht, The Netherlands) controlled with GPES software (v4.6, Eco Chemie) running on a PC.
Rotating disc electrode experiments
The rotating disc electrode (RDE) was a glassy carbon electrode (6.1204.000, Metrohm, Buckingham, UK) 3 mm in diameter controlled with a variable speed (500–3000 rpm) electrode rotator (Part No. 628-10, Metrohm). The disc electrode was polished with an alumina slurry (0.3 μm Al2O3, Metrohm) before use. A standard (12 mm in diameter) glass double junction Ag/AgCl/KCl (3M) reference electrode was used (CR4/DJ/AG, Thermo Electron, Auchtermuchty, Fife, UK) with a Pt wire auxiliary 0.5 mm in diameter (99.9%, Aldrich, Gillingham, UK). The electrodes were mounted in a small-volume 50-ml beaker used as an electrochemical cell.
Flow cell experiments
A gravity-fed flow system was used comprising a reservoir formed from a disposable syringe body (20 ml), and narrow-bore PTFE tubing (0.032 in. i.d., Lee Products, Gerrards Cross, UK). The flow was controlled with a manual two-way valve (Part No. 2420, Omnifit, Cambridge, UK). Barbed (1/16″) polypropylene female Luer fittings (Cole-Parmer, Hanwell, London, UK) enabled connection of the flow system to the injection-moulded flow cell. Electrical connection to the Autolab electrochemical system was made using crocodile clips.
Chip fabrication
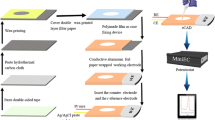
The injection-moulded flow cells were produced in-house. Figure 1 depicts the fabricated device showing the three integrated electrodes (WE, reference and auxiliary). Solid models created using AutoCAD (Mechanical Desktop 2004, Autodesk, San Jose, CA, USA) were converted into macro commands for a CNC milling machine (Datron CAT3D M6, Datron Technology, Milton Keynes, UK) to produce the milled aluminium moulds for the injection-moulding machines (Babyplast 6/6 or 6/10, Cronoplast SL, Barcelona, Spain). A four-stage process was required to manufacture a ready-to-use device. The polymer electrodes were injection moulded (moulding temperature of 220 °C) from a conducting polymer (40% carbon-fibre-filled high-impact polystyrene (HIPS), RTP 487, RTP Company (UK) Plastics Ltd., Bury, UK). The reference electrodes were coated with Ag (Ag evaporation slug, 99.99%, Aldrich) using an e-beam evaporator (Auto500 with EB3 e-beam unit, Edwards High Vacuum International, Crawley, West Sussex, UK) to a thickness of 125 nm. The WE, reference and auxiliary electrodes were then incorporated into the flow cell by an overmoulding procedure. The mould cavity for the flow cell design comprises a base plate into which the preformed electrodes are inserted in the appropriate recesses and a top plate with a relief pattern that forms the flow channel and integrated fluidic connectors. The flow cell was moulded (moulding temperature of 220 °C) from crystal polystyrene (Northern Industrial Plastics Ltd. Chadderton, UK). Using a 500-W ultrasonicwelder (Mini delta 9500, FFR Ultrasonics Ltd., Queniborough, Leicestershire, UK), with a weld time of 1 s and a hold time of 2 s, the top and base plates were sealed together to form a complete and ready-to-use device. The flow channel dimensions were 1 mm wide, 1 mm deep and 30 mm long. The working and auxiliary electrodes were 5 mm wide, the reference was 2 mm wide and the spacing between them was 2 mm.
The injection-moulded electrochemical micro flow cell. Here (A) is the Ag-coated electrode, which forms the reference (Ag/AgCl) element; (B) is the working electrode, on which the bismuth film electrode is formed; (C) is the auxiliary electrode; (D) is the device inlet and (E) is the device outlet. The flow channel is 1 mm × 1 mm and 30 mm long
Reagents and solutions
All aqueous solutions were prepared using >18MΩ water (Elga Maxima Ultra Pure, Vivendi, High Wycombe, UK). The acetate buffer was prepared from acetic acid (glacial 99.8% AnalaR grade, BDH, Poole, UK) and sodium acetate (99+% ACS grade, Aldrich, Gillingham, UK). The ammonium buffer was prepared from ammonium chloride (99.8% AnalaR grade, BDH) and ammonium hydroxide (28–30% NH3 ACS grade, Aldrich). Bismuth plating solutions were prepared with bismuth(III) nitrate pentahydrate (99.999%, Aldrich,) either in acetate buffer or with lithium bromide (99+%, Aldrich) in hydrochloric acid (1 M volumetric standard, Riedel-de Haën, Gillingham, UK), as described in the “Procedure” section. The mercury plating solution was prepared in nitric acid from a 1000 ppm mercury(II) chloride (98+%, Aldrich) stock solution, as described in the “Procedure” section. Sample solutions were made from cobalt (995 μg/l, Aldrich) and nickel (1 g/l, Fluka, Gillingham, UK) atomic absorption standards with dimethylglyoxime (99+%, Acros, Loughborough, UK) and sodium nitrite (97+%, Aldrich). The saturated AgCl solution was prepared from silver nitrate (99.9% AnalaR grade, BDH) in potassium chloride (99.5% AnalaR grade, BDH). Nitric acid (2 M volumetric standard, Riedel-de Haën) was used for electrode cleaning, as described in the “Procedure” section.
Procedure
Formation of the Ag/AgCl miniaturised reference electrode
Using an external auxiliary platinum wire located in the inlet of the microdevice, the Ag electrode was coated in AgCl (in saturated KCl solution that was also saturated with AgCl through the addition of a small quantity of AgNO3) at 2 V for 3 s.
Ex situ electroplating of the BiFE on the rotating disc electrode (RDE)
Two electroplating solutions were investigated, (A) and (B).
-
(A)
Acetate buffer Bi(III) plating solution. 100 ppm Bi(III) in 1 M acetate buffer (pH 4.5). Plated for 5 mins at −1.0 V in a quiescent solution.
-
(B)
LiBr/ HCl Bi(III) plating solution. 0.02 M Bi(III) in 0.5 M LiBr and 1 M HCl. Plated for 60 s at −0.28 V in a quiescent solution.
The RDE was then carefully rinsed before transferring it to the analysis solution.
In-line electroplating of the BiFE on the flow cell working electrode
Two electroplating solutions were investigated, (A) and (B).
-
(A)
Acetate buffer Bi(III) plating solution. 100 ppm Bi in 0.1 M acetate buffer (pH 4.5). Plated for 5 mins at −1.0 V at a flow rate of 37.1 μl/s.
-
(B)
LiBr/ HCl Bi(III) plating solution. 0.02 M Bi(III) in 0.5 M LiBr and 1 M HCl. Plated for 120 s at −0.28 V at a flow rate of 37.1 μl/s.
After formation of the BiFE, the plating flow stream was flushed with water and switched to the analysis stream.
Bismuth film cleaning procedure
The Bi(III) film was cleaned of the remaining adsorbed complexes by holding the electrode at a potential of −1.3 V for 30 s in 0.1 M HCl.
Bismuth film removal
Bi(III) films were removed by applying a conditioning potential at +0.3 V, −0.5 V and 0 V for 2 × 50 s in a flowing solution of 0.1 M HNO3.
In-line plating of MFE on the flow cell working electrode
The Hg plating solution was 200 ppm in 0.1 M HNO3. Plated for 120 s at −1 V at a flow rate of 9 μl/s. After film formation the cell was flushed with water and switched to the analysis stream.
Mercury film cleaning procedure
The film was cleaned at a potential of −1.4 V for 10 s in 0.1 M HNO3.
Mercury film removal
Films were removed at +0.8 V in a flowing solution of 0.1 M HNO3 for 60 s.
Results and discussion
The in-line prepared BiFE was assessed for its suitability in performing CAdSV of Co(II) and Ni(II). Various parameters, such as flow rates, solution composition, plating parameters and square-wave conditions, were studied to investigate the electrochemical behaviour of the Bi(III) films prepared on the injection-moulded carbon fibre electrodes.
Investigation of the effect of flow rate during the plating and accumulation stages
Using the acetate buffer-based plating solution, i.e. the 100 ppm Bi(III) in 0.1 M acetate buffer, the effect of varying the flow rate during BiFE plating procedure was examined at 20, 25 and 37 μl/s by varying the height of the reservoir in the gravity-fed flow system (with sample conditions as given in Fig. 2). The maximum response was obtained for the stripping peaks at a flow rate of 37 μl/ s and so this rate was subsequently employed in this study.
Influence of flow rate during the accumulation stage on the Ni(II) and Co(II) CAdSV peaks, where the flow rates are: (a) 5.8, (b) 15.7, (c) 25.6, (d) 37.1, (e) 42.9 and (f) 55.5 μl/s respectively. Carried out with a 10 ppb sample of Co(II) and Ni(II) in 0.1 M ammonium buffer (pH 9.2) with 0.1 mM DMG and 0.5 M NaNO2. SW conditions: Conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 5 s, pulse frequency 15 Hz, pulse amplitude 40 mV and step potential 4 mV. Scan: initial −0.8 V to end potential −1.3 V. Data were smoothed with a level-2 Savitzky–Golay filter
The effect of varying the flow rate during the accumulation stage on the peak heights of Co(II) and Ni(II) was studied over the range of 5.8–55.5 μl/s; the results are depicted in Fig. 2. The response for Co(II) increased linearly with increasing flow rate with a slope of −0.036 μAs/ μl and an intercept of −1.26 μA with a correlation coefficient (R 2 = 0.96), whereas that for Ni(II) did not show significant variation. A flow rate of 37 μl/s was selected, as this gave an acceptable response without adverse peak broadening and was compatible with the BiFE plating step flow rate, thereby simplifying the instrumentation.
Effect of composition of the BiFE plating solution
Reported methods for AdSV of Co(II) and Ni(II) have generally employed acetate buffer (pH 4.5) containing 100 mg/l Bi(III) [15, 24, 26, 28]. However, Królicka et al. [29, 30] suggested the use of a bromide-modified plating solution. Hence, both plating solutions were assessed using the RDE and flow cell setups. Figure 3 depicts the differing performances of the two plating solutions. The LiBr-containing plating solution gave the best stripping responses on both the RDE and the flow cell and was therefore used for subsequent studies.
Comparison of different Bi(III) plating solutions. a Effect of (a) 100 ppm Bi(III) in acetate buffer (1 M, pH 4.5) and (b) 0.02 M Bi(III) in 0.5 M LiBr- and 1M HCl-based preplating solutions on RDE at 500 rpm. b Effect of (c) the acetate buffer (0.1 M, pH 4.5) and (d) LiBr/HCl preplating solutions on the flow cell. c Comparision of acetate buffer pH 4.5 Bi(III) preplating solution on (e) flow cell (0.1 M) and (f) RDE (1 M). d Comparison of LiBr preplating solution on (g) flow cell and (h) RDE. Carried out with a 10 ppb sample of Co(II) and Ni(II) in 0.1 M ammonium buffer (pH 9.2) with 0.1 mM DMG and 0.5 M NaNO2. SW conditions: Conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 10 s, pulse frequency 25 Hz, pulse amplitude 50 mV and step potential 5 mV (except trace (e) which was at 15 Hz, 40 and 4 mV respectively). Scan: initial −0.8 V to end potential −1.3 V
Effect of solution parameters on the system behaviour
Buffer concentration
The effect of changing the ammonium buffer concentration over the range 0–0.5 M is shown in Fig. 4a. Increasing the buffer concentration over the range 0–0.1 M had a marked effect on the height of the Co(II) peak, but increasing the concentration further did not improve the peak height due to the increased background signal. As Co(II) and Ni(II) were sufficiently resolved at 0.1 M, this concentration was selected for all further experiments.
Effect of solution parameters on the CAdSV behaviour of a 10 ppb Co(II) and Ni(II) sample: (a) ammonium buffer (pH 9.2) concentration over the range 0–0.5 M, (b) DMG concentration over the range 0–0.35 M and (c) nitrite concentration over the range 0–0.8 M. Supporting electrolyte conditions were: (b and c) 0.1 M ammonium buffer (pH 9.2), (a and c) 0.1 mM DMG and (a and b) 0.5M NaNO2. SW conditions were: conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 10 s, pulse frequency 25 Hz, pulse amplitude 40 mV (except c: 50 mV) and step potential 4 mV (except c: 5 mV). Scan: initial −0.8 V to end potential −1.3 V
Effect of DMG concentration
Figure 4b shows the effect of increasing DMG concentration, over the range 0.01–0.35 mM, on the peak current. The Co(II) signal increases almost linearly with increasing DMG concentrations, whereas the signal for Ni(II) steadily decreased to 0.2 mM whereupon it levelled off. The signal for the Co(II) peak shifted to a slightly more negative potential (−4 mV) than that for Ni(II) with increasing concentration. A concentration of 0.3 mM was employed in the subsequent studies.
Effect of nitrite concentration
Figure 4c shows the influence of nitrite concentration on the sample signal. The Co(II) signal shows a rapid increase in signal to 0.3 M and a slower increase thereafter, whereas the Ni(II) peak height remained level to a concentration of 0.6 M. Concentrations of greater than 0.6 M lead to increased background current and so a nitrite concentration of 0.5 M was subsequently employed. Figure 5 shows the results obtained from the addition of nitrite to the sample system. Addition of nitrite to separate samples of Ni(II) and Co(II) results in increases in response for both metals, Fig. 5a and b, whereas the addition of nitrite to a mixture of 10 ppb Co(II) and Ni(II) results in an almost unchanged Ni(II) peak height whilst that for Co(II) has increased elevenfold, Fig. 5c. Figure 5d shows the reduction (16.6%) of the Co(II) peak height upon the addition of 10 ppb Ni(II), while Fig. 5e displays the reduction (51.9%) of the Ni(II) peak height upon the addition of 10 ppb Co(II).
a Effect of nitrite on stripping peak for 10 ppb Ni(II) when (a) no NaNO2 is present; (b) 0.5 M NaNO2 is present. b Effect of nitrite on stripping peak for 10 ppb Co(II) when (c) no NaNO2 is present; (d) 0.5 M NaNO2 is present. c Effect of nitrite on the stripping peaks for 10 ppb Co(II) and Ni(II) when (e) no NaNO2 is present; (f) 0.5 M NaNO2 is present. d Effect of addition of 10 ppb Ni(II) on the peak height for 10 ppb Co(II) in the presence of 0.5 M NaNO2 when (h) Co(II) only is present and (g) both Co(II) and Ni(II) are present. e Effect of addition of 10 ppb Co(II) on the peak height for 10 ppb Ni(II) in the presence of 0.5 M NaNO2 when (j) Ni(II) only is present and (k) Co(II) and Ni(II) are present. Carried out in 0.1 M ammonium buffer (pH 9.2) with 0.1 mM DMG. SW conditions: conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 5 s, pulse frequency 25 Hz, pulse amplitude 50 mV and step potential 5 mV. Scan: initial −0.8 V to end potential −1.3 V
Effect of varying the square-wave (SW) stripping parameters
Frequency
The effect of frequency was studied in the range 12.5–100 Hz, Fig. 6a depicts the results obtained. The peak heights for both metals increased with increasing SW frequency (as expected from increasing the effective scan rate) and shifted towards more negative values. However, increasing the frequency leads to insufficient time for the background current to decay and leads to an increase in the contribution to the signal and hence a distortion in peak shape. A frequency of 25 Hz was generally the maximum employed.
Effect of varying the SW stripping parameters on the CAdSV peaks of a 10 ppb Co(II) and Ni(II) sample. The variables are (a) frequency (12.5–100 Hz), (b) step potential (0.15–7 mV), (c) amplitude (5–70 mV), (d) deposition potential (−0.3 to −0.9 V), (e) deposition time (30–210 s). Supporting electrolyte: 0.1 M ammonium buffer (pH 9.2) with 0.1 mM DMG and 0.5 M NaNO2. SW conditions were (unless being investigated as above): conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 5 s, frequency 25 Hz, pulse amplitude 50 mV (except b and e: 40 mV) and step potential 5 mV (except e: 4 mV). Scan: initial −0.8 V to end potential −1.3 V
Step potential
Figure 6b shows the effect of the step potential on the peak heights. The response for Co(II) rose rapidly with rising step potentials (i.e. with an increase in effective scan rate) up to 3 mV and thereafter levelled off, whereas the response for Ni(II) increased slowly to 7 mV. Peak resolution remained the same across this step potential range, with both peaks shifting towards more negative potentials. Values of 4 or 5 mV were generally found to be ideal for adequate peak definition.
Amplitude
The effect of the SW pulse amplitude was studied over the range 5–80 mV. Both peaks shifted to more positive values, see Fig. 6c and they both initially increased rapidly in height (5–20 mV) whereupon the responses for Co(II) and Ni(II) levelled off as a result of the increase in contribution from the background current. A SW pulse amplitude of either 40 or 50 mV was employed.
Deposition potential
Figure 6d shows the influence of the deposition potential on the peak currents. At potentials less than −0.4 V the Ni(II) signal is completely suppressed and the Co(II) signal was markedly reduced due to the oxidation of the bismuth film (at around −0.46 V in alkaline solutions). Both peaks gradually increase with potential in the range of −0.4 V to a maximum at −0.8 V and then exhibit a sharp reduction in adsorption at a more negative potential of −0.9 V resulting from further reduction of the analyte complex. A deposition potential of −0.8 V was selected, as this gave the maximum response for both metals.
Accumulation time
The influence of the accumulation time on the stripping signal was examined. Figure 6e depicts the response in peak currents for 10 ppb each of Ni(II) and Co(II). For both Co(II) and Ni(II) the response increases with accumulation time up to 150 s for Co(II) and 180 s for Ni (II), after which the response levels off, indicating saturation of the surface. An accumulation time of 120 s was used for subsequent measurements as this gave the best compromise between sensitivity and analysis time.
Analytical characterisation
The simultaneous determination of Co(II) and Ni(II) over the concentration range of 1–20 ppb was investigated. The determination was carried out in 0.1 M ammonium buffer (pH 9.2) with 0.1 mM DMG and 0.5 M NaNO2. SW conditions: conditioning potential −1.3 V (10 s), deposition potential −0.8 V (120 s), equilibration time 5 s, pulse frequency 25 Hz, pulse amplitude 50 mV and step potential 5 mV. Scan: initial −0.8 V to end potential −1.3 V. The BiFE exhibited a well-defined response to changing concentration and the peak current response (y, μA) was linear over the concentration range (x, ppb) of 1–20 ppb, as expressed in the calibration plots for Co(II),y = −11.4x − 85.5 (R 2 = 0.96), and Ni(II),y = −1.64x – 7.20 (R 2 = 0.92), respectively.
The stability of the BiFE formed on the injection-moulded substrates was investigated by performing replicate runs on the same film. Ten replicate runs gave an average peak height for Ni(II) of −1.05 ± 0.10 × 10−5 A (rsd: 9.6%) and for Co(II) −8.19 ± 0.82 × 10−5 A (rsd: 10.0%).
To compare the working electrode behaviour, AdSV and CAdSV of Co(II) and Ni(II) were also performed at a MFE in the flow cell. The solutions needed to be deaerated before analysis on the MFE. Neither AdSV or CAdSV of Co(II) on a MFE was possible in these particular flow cells due to a significant shift in the stripping peak to an extreme cathodic potential (<−1.4 V vs Ag/AgCl), which led to analytical uncertainty at the edge of the supporting electrolyte potential window. However, AdSV of Ni(II) was possible, as illustrated in Fig. 7. It has been noted previously by this group [57, 58] that the main drawback with MFEs is the difficulty involved in regenerating the active mercury surface after use, necessitating the inclusion of cleaning and mercury film renewal procedures. In contrast to the MFEs, the BiFEs formed in these flow cells enabled the generation of reproducible, quantitative analytical data, due to the enhanced stability of the bismuth films during the cleaning cycles, and the reduced cathodic potential for the cobalt stripping peak.
AdSV of Ni(II) on a mercury film electrode. Here decimal numerals indicate the film number and roman numerals indicate replicate analyses carried out on the same film. Carried out with a 200 ppb sample in 0.1 M ammonium buffer (pH 9.3) with 0.1 mM DMG (deaerated) at a flow rate of 16 μl/s. SW conditions: conditioning potential −1.4 V (10 s), deposition potential −0.5 V (200 s), equilibration time 5 s, pulse frequency 25 Hz, pulse amplitude 10 mV and step potential 4 mV. Scan: −0.5 V to end potential −1.4 V. Data were smoothed with a level-2 Savitzky–Golay filter
Conclusions
The application of a BiFE for the determination of Co(II) and Ni(II) by square-wave CAdSV under flow conditions in a non-deaerated solution has been demonstrated. The injection-moulded carbon-fibre-filled polystyrene electrodes have proven to be suitable supports for the formation of bismuth films. The LiBr-containing plating solution was found to offer superior performance for both the RDE and the flow cell under the alkaline conditions required for CAdSV. The application of a flow stable BiFE in an inexpensive sensor system permits the possibility of autonomous on-site industrial and environmental monitoring. The inherent disposability of the devices coupled with their mercury-free status should advance the scope of the sensor to point-of-care clinical applications.
References
Bonfil Y, Brand M, Kirowa-Eisner E (1999) Anal Chim Acta 387:85–95
Bonfil Y, Brand M, Kirowa-Eisner E (2002) Anal Chim Acta 464:99–114
Bonfil Y, Kirowa-Eisner E (2002) Anal Chim Acta 457:285–296
Nolan MA, Kounaves SP (1999) Anal Chem 71:3567–3573
Brainina KZ, Kubysheva IV, Miroshnikova EG, Parshavkov SI, Maksimov YG, Volkonsky AE (2001) Field Anal Chem Technol 5:260–271
Wagner K, Strojek JW, Koziel K (2002) Electroanalysis 15:392–397
Honeychurch KC, Hart JP (2003) TrAC 22:456–469
Wang J, Lu J, Hočevar SB, Farias PAM, Ogorevc B (2000) Anal Chem 72:3218–3222
Swedish Chemicals Inspectorate (KEMI) (2004) Mercury: investigation of a total ban (KEMI report No 4/04). KEMI, Stockholm. http://www.kemi.se/upload/Trycksaker/Pdf/Rapporter/Rapport4_04.pdf, accessed 23rd October 2007
European Commission (2005) Commision proposes strategy to deal with mercury pollution including a ban on exports (Press Release IP/05/114). EC, Brussels. http://europa.eu.int/rapid/pressReleasesAction.do?reference=IP/05/114&format=HTML&aged=0&language=EN&guiLanguage=en, accessed 23rd October 2007
European Parliament, Committee on the Environment, Public Health and Food Safety (2007) Mercury: ban exports and make storage safer (Press Release REF.: 20070502IPR06205). European Parliament, Strasbourg. http://www.europarl.europa.eu/news/expert/infopress_page/064-6215-122-05-18-911-20070502IPR06205-02-05-2007-2007-false/default_en.htm, accessed 23rd October 2007
Wittstock G, Strübing A, Szargan R, Werner G (1998) J Electroanal Chem 444:61–73
Hočevar SB, Ogorevc B, Wang J, Pihlar B (2002) Electroanalysis 14:1707–1712
Baldo MA, Daniele S, Bragato C (2003) J Phys (Paris) IV 107:103–106
Wang J, Lu J (2000) Electrochem Commun 2:390–393
Wang J, Lu J, Kirgöz UA, Hočevar SB, Ogorevc B (2001) Anal Chim Acta 434:29–34
Hutton EA, Ogorevc B, Hočevar SB, Weldon F, Smyth MR, Wang J (2001) Electrochem Commun 3:707–711
Wang J, Kirgöz UA, Lu J (2001) Electrochem Commun 3:703–706
Wang J, Deo RP, Thongngamdee S, Ogorevc B (2001) Electroanalysis 13:1153-1156
Hočevar SB, Wang J, Deo RP, Ogorevc B (2002) Electroanalysis 14:112–115
Lin L, Lawrence NS, Thongngamdee S, Wang J, Lin Y (2005) Talanta 65:144–148
Kefala G, Economou A, Voulgaropoulos A, Sofoniou M (2003) Talanta 61:603–610
Kefala G, Economou A, Voulgaropoulos A (2004) Analyst 129:1082–1090
Morfobos M, Economou A, Voulgaropoulos A (2004) Anal Chim Acta 519:57–64
Chatzitheodorou E, Economou A, Voulgaropoulos A (2004) Electroanalysis 16:1745–1754
Hutton EA, Hočevar SB, Ogorevc B, Smyth MR (2003) Electrochem Commun 5:765–769
Hutton EA, Ogorevc B, Smyth MR (2004) Electroanalysis 16:1616–1621
Hutton EA, van Elteren JT, Ogorevc B, Smyth MR (2004) Talanta 63:849–855
Krolicka A, Bobrowski A, Kalcher K, Mocak J, Švancara I, Vytřas K (2003) Electroanalysis 15:1859–1863
Królicka A, Bobrowski A (2004) Electrochem Commun 6:99–104
Maghasi AT, Halsall HB, Heineman WR, Rodriguez Rilo HL (2004) Anal Biochem 326:183–189
Guo Z, Feng F, Hou Y, Jaffrezic-Renault N (2005) Talanta 65:1052–1055
Demetriades D, Economou A, Voulgaropoulos A (2004) Anal Chim Acta 519:167–172
Krolicka A, Pauliukaite R, Švancara I, Metelka R, Bobrowski A, Norkus E, Kalcher K, Vytřasfs K (2002) Electrochem Commun 4:193–196
Vytřas K, Švancara I, Metelka R (2002) Electroanalysis 14:1359–1364
Flechsig G-U, Korbout O, Hočevar SB, Thongngamdee S, Ogorevc B, Gründler P, Wang J (2002) Electroanalysis 14:192–196
Wang J, Lu J, Hočevar SB, Ogorevc B (2001) Electroanalysis 13:13–16
Kadara RO, Tothill IE (2004) Anal Bioanal Chem 378:770–775
Banks CE, Kruusma J, Moore RR, Tomčík P, Peters J, Davis J, Komorsky-Lovric Š, Compton RG (2005) Talanta 65:423–429
Pauliukaite R, Metelka R, Švancara I, Królicka A, Bobrowski A, Vytřas K, Norkus E, Kalcher K (2002) Anal Bioanal Chem 374:1155–1158
Pauliukaite R, Hočevar SB, Ogorevc B, Wang J (2004) Electroanalysis 16:719–723
Economou A (2005) TrAC 24:334–340
Charalambous A, Economou A (2005) Anal Chim Acta 547:53–58
Prior C, Walker GS (2006) Electroanalysis 18:823–829
Yong L, Armstrong KC, Dansby-Sparks RN, Carrington NA, Chambers JQ, Xue Z-L (2006) Anal Chem 78:7582–7587
Lin L, Thongngamdee S, Wang J, Lin Y, Sadik OA, Ly S-Y (2005) Anal Chim Acta 535:9–13
Kefala G, Economou A, Voulgaropoulos A (2006) Electroanalysis 18:223–230
Kefala G, Economou A, Sofoniou M (2005) Talanta 68:1013–1019
Wang J, Lu D, Thongngamdee S, Lin Y, Sadik OA (2005) Talanta 69:914–917
Wang J, Thongngamdee S, Lu D (2006) Electroanalysis 18:59–63
Kefala G, Economou A (2006) Anal Chim Acta 576:283–289
Economou A, Voulgaropoulos A (2007) Talanta 71:758–765
Vega M, van den Berg CMG (1997) Anal Chem 69:874–881
Colombo C, van den Berg CMG (1997) Anal Chim Acta 337:29–40
Bobrowski A, Zarębski J (2000) Electroanalysis 12:1177–1186
Mylonakis A, Economou A, Fielden PR, Goddard NJ, Voulgaropoulos A (2004) Electroanalysis 16:524–531
Economou A, Fielden PR (1997) TrAC 16:286–292
Economou A, Fielden PR (2003) Analyst 128:205–212
Acknowledgements
The authors would like to express their thanks to the following for financial support: EPSRC (SJB), Petroleum University of Technology, Ahwaz, Iran (NGN) and the University of Athens, Greece (AE).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gharib Naseri, N., Baldock, S.J., Economou, A. et al. Disposable electrochemical flow cells for catalytic adsorptive stripping voltammetry (CAdSV) at a bismuth film electrode (BiFE). Anal Bioanal Chem 391, 1283–1292 (2008). https://doi.org/10.1007/s00216-008-1948-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1948-5