Abstract
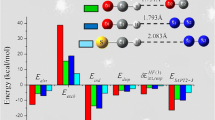
Interactions of group 12 metal(II) species (Hg2+, Cd2+, Zn2+, Hg(H2O) 2+ n , Cd(H2O) 2+ n , and Zn(H2O) 2+ n (n = 1, 2) with neutral (RSH), deprotonated (RS−), and doubly deprotonated cysteine species (abbreviated as “H2cys”, “Hcys−”, and “cys2−”, respectively) are examined with the Becke three-parameter Lee–Yang–Parr (B3LYP) hybrid functional after preliminary screening in a conformation analysis with the Parameterized Model number 3 (PM3) semiempirical method. Effects of water on aqueous solution are evaluated by microsolvation and polarized continuum model (PCM) approaches. In the most stable conformations of M(H2cys)2+ and M(Hcys)+ complexes (M = Hg2+, Cd2+, and Zn2+), the SH group of the cysteine moiety is already deprotonated and undergoes strong binding with the metal ion. Among Hg(H2cys)2+ complexes, cysteine complexes of Hg2+ without deprotonation of the SH group and mercury(II) carboxylato-type structures are at least 83 and 117 kJ/mol less stable in energy than the most stable complex (B3LYP/6-311++G(d,p)-SDD+d+f//B3LYP/6-31G(d)-SDD+d). Although Zn2+ binds more strongly than Hg2+ to a H2cys molecule at the high-level CCSD(T)/6-311++G(d,p)-SDD+d+f//B3LYP/6-311++G(d,p)-SDD+d+f level, [Hg(H2O)2]2+ is stronger than [Zn(H2O)2]2+ because the deformation of [Zn(H2O)2]2+ required to bind to cys is much more than in [Hg(H2O)2]2+. Complexes with a deprotonated cysteine, M(Hcys)+ and M(cys), prefer a multidentate structure.



















Similar content being viewed by others
Notes
A part of the present studies are already orally communicated.
References
Zalups RK, Koropatnick J (eds) (2000) Molecular biology and toxicology of metals. Taylor & Francis, London
Walsh CT, Distefano MD, Moore MJ, Shewchuk LM, Verdine G (1988) FASEB J 2:124–130
Klaassen CD (ed) (2001) Casarett and Doull’s toxicology, 6th edn
Steele RA, Opella SJ (1997) Biochemistry 36:6885–6895
Lafrance-Vanasse J, Lefebvre M, Di Lello P, Sygusch J, Omichinski JG (2009) J Biol Chem 284:938–944
de Montellano PRO (ed) (2005) Cytochrome P450. Kluwer/Academic/Plenum, New York
Choe Y-K, Nagase S (2005) J Comput Chem 26:1600–1611
Yanai TK, Mori S (2008) Chem Asian J 3:1900–1911
Yanai TK, Mori S (2009) Chem Eur J 15:4464–4473
Chan J, Huang Z, Merrifield ME, Salgado MT, Stillman MJ (2002) Coord Chem Rev 233–234:319–339
Henkel G, Krebs B (2004) Chem Rev 104:801–824
Stillman MJ, Shaw CF III, Suzuki KT (eds) (1991) Metallothioneins. VCH, New York
Zalups RK, Barfuss DW (1996) Toxicology 109:15–29
Oyama Y, Nakata M, Sakamoto M, Chikahisa L, Miyoshi N, Satoh M (1998) Environ Toxicol Pharmacol 6:221–227
Zalups RK, Barfuss DW (1995) J Toxicol Environ Health 44:401–413
Bridges CC, Zalups RK (2010) J. Toxicol Environ Health Part B 13:385–410
Clarkson TW, Magos L (2006) Crit Rev Toxicol 36:609–662
Aschner M, Clarkson TW (1988) Brain Res 462:31–39
Hirayama K (1980) Toxicol Appl Pharmocol 55:318–323
Kerr KA, Ashmore JP, Koetzle TF (1975) Acta Cryst B31:2022–2026
Noguera M, Rodríguez-Santiago L, Sodupe M, Bertran J (2001) J Mol Struct (THEOCHEM) 537:307–318
Fernández-Ramos A, Cabaleiro-Lagoa E, Hermida-Ramóna JM, Martínez-Núñeza E, Peña-Gallegoa A (2000) J Mol Struct (THEOCHEM) 498:191–200
Pecul M (2006) Chem Phys Lett 418:1–10
Sanz ME, Blanco S, López JC, Alonso JL (2008) Angew Chem Int Ed 47:6216–6220
Taylor NJ, Wong YS, Chieh PC, Carty AJ (1975) J Chem Soc Dalton Trans 438–442
Taylor NJ, Carty AJ (1977) J Am Chem Soc 99:6143–6145
Katono Y, Inoue Y, Chujo R (1977) Polym J 9:471–478
Natusch DFS, Porter LJ (1971) J Chem Soc A 2527–2535
Rubius FM, Verduci C, Giampiccolo R, Pulvirenti S, Brambilla G, Colombi A (2004) J Am Soc Mass Spectrom 15:288–300
Cheesman BV, Arnold AP, Rabenstein DL (1988) J Am Chem Soc 110:6359–6364
Jalilehvand F, Leung BO, Izadifard M, Damian E (2006) Inorg Chem 45:66–73
Leung BO, Jalilehvand F, Szilagyi RK (2008) J Phys Chem B 112:4770–4778
Hughes W Jr (1947) J Am Chem Soc 69:1836–1837
Nriagu JO (ed) (1979) The biogeochemistry of mercury in the environment. Elsevier/North-Holland Biomedical Press, Amsterdam
Oram PD, Fang X, Fernando Q, Letkeman P, Letkeman D (1996) Chem Res Toxicol 9:709–712
Fuhr BJ, Rabenstein DL (1973) J Am Chem Soc 95:6944–6950
Mah V, Jalilehvand F (2010) Chem Res Toxicol 23:1815–1823
Tarbouriech N, Curran J, Ruigrok RWH, Burmeister WP (2000) Nat Struct Biol 7: 777–781. 1EZJ in Protein Data Bank
Zalups RK, Barfuss DW (2002) J Toxicol Environ Health Part A 65:1471–1490
Martelli A, Rousselet E, Dyeke C, Bouron A, Moulis J-M (2006) Biochimie 88:1807–1814
Bottari E, Festa MR (1997) Talanta 44:1705–1718
Jalilehvand F, Mah V, Leung BO, Mink J, Bernard GM, Hajba L (2009) Inorg Chem 48:4219–4230
Barglik-Chory C, Remenyi C, Strohm H, Müller G (2004) J Phys Chem B 108:7637–7640
Cai Z-X, Yang H, Zhang Y, Yan X-P (2006) Anal Chim Acta 559:234–239
Plapp BV, Eklund H, Jones TA, Branden CI (1983) J Biol Chem 258:5537–5547
Ramaswamy S, Eklund H, Plapp BV (1994) Biochemistry 33:5230–5237
Agarwal PK, Webb SP, Hammes-Schiffer S (2000) J Am Chem Soc 122:4803–4812
Christianson DW, Lipscomb WN (1986) Proc Natl Acad Sci USA 83:7568–7572
Cameron AD, Ridderstrom M, Olin B, Kavarana MJ, Creighton DJ, Mannervik B (1999) Biochemistry 38:13480–13490
Venugopal B, Luckey TD (1978) Metal toxicity in mammals 2, chemical toxicity of metals and metalloids. Plenum Press, New York, USA
Cerda BA, Wesdemiotis C (1995) J Am Chem Soc 117:9734–9739
Hoyau S, Ohanessian G (1997) J Am Chem Soc 119:2016–2024
Zimmermann T, Zeizinger M, Burda JV (2005) J Inorg Biochem 99:2184–2196
Spezia R, Tournois G, Cartailler T, Tortajada J, Jeanvoine Y (2006) J Phys Chem A 110:9727–9735
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Belcastro M, Marino T, Russo N, Toscano M (2005) J Mass Spectr 40:300–306
Mori S, Kishi T, Endoh T (2004) 30th annual meeting of the society of toxicology of Japan, Sagamihara, Japan
Mori S, Kishi T, Endoh T, Sudou K (2004) 84th annual meeting of Chemical Society of Japan, Nishinomiya, Japan
Mori S, Endoh T, Kishi T (2004) 7th international conference on mercury as a global pollutant, Ljubljana, Slovenia
Hoffmeyer RE, Singh SP, Doonan CJ, Ross ARS, Hughes RJ, Pickering IJ, George GN (2006) Chem Res Toxicol 19:753–759
Bridges CC, Zalups RK (2006) Chem Res Toxicol 19:1117–1118
Hoffmeyer RE, Singh SP, Doonan CJ, Pickering IJ, George GN, Ross ARS, Hughes RJ (2006) Chem Res Toxicol 19:1118–1120
Krupp EM, Miline BF, Mestrot A, Meharg AA, Feldmann J (2008) Anal Bioanal Chem 390:1753–1766
Bergner A, Dolg M, Kuechle W, Stoll H, Preuss H (1993) Mol Phys 80:1431–1441
Ramírez J-Z, Vargas R, Garza J, Hay BP (2006) J Chem Theory Comput 2:1510–1519
Gourlaouen C, Piquemak J-P, Saue T, Parisel O (2005) J Comput Chem 27:142–156
Stricks W, Kolthoff IM (1953) J Am Chem Soc 75:5673–5681
Walker MD, Williams DR (1974) J Chem Soc Dalton Trans 1186–1189
Lenz GR, Martell AE (1964) Biochemistry 3:745–750
Smith RM, Martell AE (2003) NIST critically selected stability constants of metal complexes database, Version 7.0
Bottari E, Festa MR (1997) Talanta 44:1705–1718
Starý J, Kratzer K (1988) J Radioanal Nucl Chem Lett 126:69–75
Berthon G (1995) Pure Appl Chem 67:1117–1240
Rulíšek L, Havlas Z (2002) J Phys Chem A 106:3855–3866
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem B 111:9146–9152
Tai H-C, Lim C (2006) J Phys Chem A 110:452–462
Dimakis N, Farooqi MJ, Garza ES, Bunker G (2008) J Chem Phys 128:115104
Ishimori K-i, Mori S, Ito Y, Ohashi K, Imura H (2009) Talanta 78:1272–1279
Marino T, Toscano M, Russo N, Grand A (2006) J Phys Chem B 110:24666–24673
Dudev T, Lim C (2007) J Am Chem Soc 129:12497–12504
Schmitt M, Böhm M, Ratzer C, Vu C, Kalkman I, Meerts WL (2005) J Am Chem Soc 127:10356–10364
Ahn D-S, Park S-W, Jeon I-S, Lee M-K, Kim N-H, Han Y-H, Lee S (2003) J Phys Chem B 107:14109–14118
Michaux C, Wouters J, Perpete EA, Jacquemin D (2008) J Phys Chem B 112:9896–9902
Bachrach SM, Nguyen TT, Demoin DW (2009) J Phys Chem A 113:6172–6181
Moisés CL, Ramos DR, Santaballa JA (2006) Chem Phys Lett 417:28–33
Rai AK, Fei W, Lu Z, Lin Z (2009) Theor Chem Acc 124:37–47
Gao B, Wyttenbach T, Bowers MT (2009) J Am Chem Soc 131:4695–4701
Shepler BC, Wright AD, Balabanov NB, Peterson KA (2007) J Phys Chem A 111:11342–11349
Spartan’04, Wavefuntion Inc, Irvine
See Cramer CJ (2002) Essentials of computational chemistry. Wiley, Chichester
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. John Wiley, New York
Ehlers AW, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler KF, Stegmann R, Veldkamp A, Frenking G (1993) Chem Phys Lett 208:111–114
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Takano Y, Houk KN (2005) J Chem Theory Comput 1:70–77
Gaussian 03, Revision B.03, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liashenko G Liu, Piskorz AP, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian, Inc., Wallingford
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926. And references cited therein
Bondi A (1964) J Phys Chem 68:441–451
Pearson PG (1963) J Am Chem Soc 85:3533–3539
Richens DT (1997) The chemistry of aqua ions. Wiley, Chichester
Eisler R, Hennekey RJ (1977) Arch Environ Contam Toxicol 6:315–323
Lewis DFV, Dobrota M, Taylor MG, Parke DV (1999) Environ Toxicol Chem 18:2199–2204
Acknowledgments
This work was supported by Grants-in-Aid No. 19550004 for Scientific Research from JSPS and by Scientific Research on Priority Areas “Molecular Theory for Real Systems”, No. 20038005 from MEXT. The generous allotment of computation time from the Research Center for Computational Science (RCCS), the National Institutes of Natural Sciences, Japan, is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Shigeru Nagase on the occasion of his 65th birthday and published as part of the Nagase Festschrift Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, S., Endoh, T., Yaguchi, Y. et al. Quantum chemical studies on the role of water microsolvation in interactions between group 12 metal species (Hg2+, Cd2+, and Zn2+) and neutral and deprotonated cysteines. Theor Chem Acc 130, 279–297 (2011). https://doi.org/10.1007/s00214-011-0975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-011-0975-z