Abstract
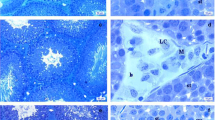
Glyphosate is a herbicide widely used to kill weeds both in agricultural and non-agricultural landscapes. Its reproductive toxicity is related to the inhibition of a StAR protein and an aromatase enzyme, which causes an in vitro reduction in testosterone and estradiol synthesis. Studies in vivo about this herbicide effects in prepubertal Wistar rats reproductive development were not performed at this moment. Evaluations included the progression of puberty, body development, the hormonal production of testosterone, estradiol and corticosterone, and the morphology of the testis. Results showed that the herbicide (1) significantly changed the progression of puberty in a dose-dependent manner; (2) reduced the testosterone production, in semineferous tubules’ morphology, decreased significantly the epithelium height (P < 0.001; control = 85.8 ± 2.8 μm; 5 mg/kg = 71.9 ± 5.3 μm; 50 mg/kg = 69.1 ± 1.7 μm; 250 mg/kg = 65.2 ± 1.3 μm) and increased the luminal diameter (P < 0.01; control = 94.0 ± 5.7 μm; 5 mg/kg = 116.6 ± 6.6 μm; 50 mg/kg = 114.3 ± 3.1 μm; 250 mg/kg = 130.3 ± 4.8 μm); (4) no difference in tubular diameter was observed; and (5) relative to the controls, no differences in serum corticosterone or estradiol levels were detected, but the concentrations of testosterone serum were lower in all treated groups (P < 0.001; control = 154.5 ± 12.9 ng/dL; 5 mg/kg = 108.6 ± 19.6 ng/dL; 50 mg/dL = 84.5 ± 12.2 ng/dL; 250 mg/kg = 76.9 ± 14.2 ng/dL). These results suggest that commercial formulation of glyphosate is a potent endocrine disruptor in vivo, causing disturbances in the reproductive development of rats when the exposure was performed during the puberty period.




Similar content being viewed by others
References
Abreu CH Jr, Muraoka T, Lavorante AF (2003) Relationship between acidity and chemicals properties of Brazilian soils. Sci Agric 60:337–343
Akingbemi BT (2005) Estrogen regulation of testicular function. Reprod Biol Endocrinol 3:1–13
Atanassova NN, Walker M, McKinnel C, Fisher JS, Sharpe RM (2005) Evidence that androgens and oestrogens, as well follicle stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol 184:107–117
Benachour N, Sipahutar H, Moslemi S, Gasnier C, Travert C, Seralini GE (2007) Time and dose-dependent effects of Roundup on human embryonic and placental cells. Arch Environ Contam Toxicol 53:126–133
Benedetti AL, Vituri CD, Trentin AG, Domingues MAC, Alvarez-Silva M (2004) The effects of sub-chronic exposure of Wistar rats to the herbicide Glyphosate-Biocarb. Toxicol Lett 153:227–232
Brausch JM, Smith PN (2007) Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch Environ Contam Toxicol 52:217–221
Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL (1997) Target disruption of the mouse gene encoding esteroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA 94:11540–11545
Cerdeira AL, Gazziero DLP, Duke SO, Matallo MB, Spadotto CA (2007) Review of potential environmental impacts of transgenic glyphosato-resistent soybean in Brazil. J Environ Sci Health B 42:539–549
Clementi M, Tiboni GM, Causin R, Rocca CL, Maranghi F, Raffagnato F, Tenconi R (2008) Pesticides and fertility: an epidemiological study in Northeast Italy and review of the literature. Reprod Toxicol 26:13–18
Colborn T, Dumanoski D, Meyers JP (1996) Our stolen future: are we threatening our fertility, intelligence and survival? A scientific detective story. Ed Dutton, New York
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Perspect 114:1803–1806
Curwin BD, Hein MJ, Sanderson WT, Nishioka MG, Reynolds SJ, Warm EM, Alavanja MC (2005) Pesticide contamination inside farm and nonfarm homes. J Occup Environ Hyg 2:357–367
Curwin BD, Hein MJ, Nishioka SandersonWT, MG ReynoldsSJ, Warm EM, Alavanja MC (2007) Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. J Occup Environ Hyg 51:53–55
Dallegrave E, Mantese FD, Oliveira RT, Andrade AJ, Dalsenter PR, Langeloh A (2007) Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol 81(9):665–673
Epand RM (2006) Cholesterol interactions of proteins with membrane domains. Prog Lipid Res 45:279–294
FAO and WHO (2004) Pesticides residues in food. Toxicological evaluations. Available via http://www.who.int/ipcs/publications/jmrp/draft_impr_2004_monograph.pdf. Accessed 14 July 2007
Foster WG, Neal MS, Han MS, Dominguez MM (2008) Environmental contaminants and human infertility: hypothesis or cause for concern? J Toxicol Environ Health 11:162–176
Getenga ZM, Kengara FO (2004) Mineralization of glifosate in compost-amended soil under control conditions. Bull Environ Contam Toxicol 72:266–275
Goldsborough LG, Brown DJ (1993) Dissipation of glyphosate and aminomethylphosphonic acid in water and sediments of boreal forest. Environ Toxicol Chem 12:1139–1147
Goyal HO, Robateou A, Braden TD, Williams CS, Srivastava KK, Ali K (2003) Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol Reprod 68:2081–2091
Hafez ESE, Hafez B (2000) Reproduction in farm animals. Ed Lippincott Williams & Wilkins, Philadelphia
Haney RL, Senseman SA, Hons FM (2002) Bioremediation and biodegradation: effect of Roundup ultra on microbial activity and biomass from selected soils. J Environ Qual 31:730–735
Hayes WJ, Laws ER (1991) Handbook of pesticide toxicology. Academic Press, San Diego
Lu FC (1995) A review of the acceptable daily intakes of pesticides assessed by WHO. Regul Toxicol Pharmacol 21:352–364
Marc J, Mulner-Lorillon O, Boulben S, Hureau D, Durand G, Bellé R (2002) Pesticide Roundup provokes cell division dysfunction at the level of CDK1/cyclin B activation. Chem Res Toxicol 15:326–331
Norman AW, Litwack G (1997) Hormones. Academic Press, California
Ojeda SR, Urbanski HF (1994) Puberty in the rat. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York
Oliveira AG, Telles LF, Hess RA, Mahecha GAB, Oliveira CA (2007) Effects of the herbicide Roundup on the epididymal region of drakes Anas platyrhynchos. Reprod Toxicol 23:182–191
Parker RM (2006) Testing for reproductive toxicity. In: Hood RD (ed) Developmental and reproductive toxicology. Taylor and Francis, New York
Peixoto F (2005) Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere 61:1115–1122
Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini G (2005) Differential effects of glyphosate and Roundup on human placental cells. Environ Health Perspect 113:716–720
Roeleveld N, Bretveld R (2008) The impact of pesticides on male fertility. Curr Opin Obstet Gynecol 20:229–233
Romano RM, Romano MA, Moura MO, Oliveira CA (2008) A exposição ao glifosato-Roundup causa atraso no início da puberdade em ratos machos. Braz J Vet Res Anim Sci 45:481–487
Scott FG (2005) Developmental biology. London: Ed Sinauer. Available via http://www.ncbi.nlm.nih.gov. Accessed 14 Oct 2008
Silva MD, Peralba MCR, Mattos MLT (2003) Determinação do glifosato e ácido aminometilfosfônico em águas superficiais do Arroio Passo do Pilão. Pest: Ecotox e Meio Amb 13:19–28
Solomon GM, Schettler T (2000) Environment and health: endocrine disruption and potential human health implications. Can Med Assoc J 163:1471–1476
Stoker TE, Parks LG, Gray LE, Cooper RL (2000) Endocrine-disrupting chemicals: pubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Endocrine disrupting screening and testing advisory committee. Crit Rev Toxicol 30:197–252
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-base formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197
Walsh LP, McCormick C, Martin C, Stocco DM (2000) Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ Health Perspect 108:769–776
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Acknowledgments
The authors would like to thank the Brazilian National Council of Scientific and Technological Development for financial support of this research (Edital MCT/CNPq 02/2006-Universal/process 471651/2006-0), the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, R.M., Romano, M.A., Bernardi, M.M. et al. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch Toxicol 84, 309–317 (2010). https://doi.org/10.1007/s00204-009-0494-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0494-z