Abstract
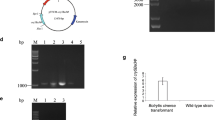
Despite the fact that Bacillus thuringiensis (Bt) is found in more than 90 % of the products used against insects, it has some difficulty reaching the internal regions where the larvae feed. To solve this problem, many genetically modified microorganisms that colonize the same pests have been developed. Thus, the endophytic bacterium Pantoea agglomerans (33.1), which has been recently described as a promising sugarcane growth promoter, was genetically modified with the pJTT vector (which carries the gene cry1Ac7) to control the sugarcane borer, Diatraea saccharalis. Firstly, the bioassays for D. saccharalis control by 33.1:pJTT were conducted with an artificial diet. A new in vivo methodology was also developed, which confirmed the partial control of larvae by 33.1:pJTT. The 33.1:pJTT strain was inoculated into sugarcane stalks containing the D. saccharalis larvae. In the sugarcane stalks, 33.1:pJTT was able to increase the mortality of D. saccharalis larvae, impair larval development and decrease larval weight. Sugarcane seedlings were inoculated with 33.1:pJTT, and re-isolation confirmed the capacity of 33.1:pJTT to continuously colonize the sugarcane. These results prove that P. agglomerans (33.1), a sugarcane growth promoter, can be improved by expressing the Cry protein, and the resulting strain is able to control the sugarcane borer.




Similar content being viewed by others
References
Araújo WL, Maccheroni W Jr, Aguilar-Vildoso CI, Barroso HOS, Azevedo JL (2001) Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can J Microbiol 47:229–236. doi:10.1139/cjm-47-3-229
Arencibia A, Vazquez RI, Prieto D, Tellez P, Carmona ER, Coego A, Hernandez L, Delariva GA, Selmanhousein G (1997) Transgenic sugarcane plants resistant to stem borer attack. Mol Breeding 3:247–255. doi:10.1023/A:1009616318854
Baldani JI, Baldani VLD, Seldin L, Döbereiner J (1986) Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol 36:86–93. doi:10.1099/00207713-36-1-86
Barboza-Corona JE, Contreras JC, Velazquez-Robledo R, Bautista-Justo M, Gomez-Ramirez M, Cruz-Camarillo R, Ibarra JE (1999) Selection of chitinolytic strains of Bacillus thuringiensis. Biotechnol Lett 21:1125–1129. doi:10.1023/A:1005626208193
Barboza-Corona JE, Nieto-Mazzocco E, Velazquez-Robledo R, Salcedo-Hernandez R, Bautista-Justo M, Jimenez B, Ibarra JE (2003) Cloning, sequencing, and expression of the chitinase gene chiA74 from Bacillus thuringiensis. Appl Environ Microbiol 69:1023–1029. doi:10.1128/AEM.69.2.1023- 1029.2003
Bonaterra A, Mari M, Casalini L, Montesinos E (2003) Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int J Food Microbiol 84:93–104. doi:10.1016/S0168-1605(02)00403-8
Bora RS, Murty MG, Shenbagarathai R, Sekar V (1994) Introduction of a lepidopteran-specific insecticidal crystal protein gene of Bacillus thuringiensis subsp. kurstaki by conjugal transfer into a Bacillus megaterium strain that persists in the cotton phyllosphere. Appl Environ Microbiol 60:214–222
Botelho PSM, Macedo N (2002) Cotesia flavipes para o controle de Diatraea saccharalis. In: Parra JRP, Botelho PSM, Corrêa-Ferreira BS, Bento JMS (eds) Controle biológico no Brasil: parasitóides e predadores. Manole, São Paulo, pp 409–425
Cavalcante VA, Döbereiner J (1988) A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 108:23–31. doi:10.1007/BF02370096
Cheavegatti-Gianotto A, Abreu HMC, Arruda P, Bespalhok-Filho JC, Burnquist WL, Creste S, Ciero L, Ferro JA, Figueira AVO, Filgueiras TS, Sá MFG, Guzzo EC, Hoffmann HP, Landell MGA, Macedo N, Matsuoka S, Reinach FC, Romano E, Silva WJ, Silva-Filho MC, Ulian EC (2011) Sugarcane (Saccharum x officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Tropical Plant Biol 4:62–89. doi:10.1007/s12042-011-9068-3
Choi YL, Gringorten JL, Belanger L, Morel L, Bourque D, Masson L, Groleau D, Miguez CB (2008) Production of an insecticidal crystal protein from Bacillus thuringiensis by the methylotroph Methylobacterium extorquens. Appl Environ Microbiol 74:5178–5182. doi:10.1128/AEM.00598-08
de Macedo CL, Martins ES, Pepino de Macedo LL, Santos AC, Praça LB, Góis LAB, Monnerat RG (2012) Selection and characterization of Bacillus thuringiensis efficient strains against Diatraea saccharalis (Lepidoptera: Crambidae) Pesq Agropec Bras 47:1759–1765. doi: 10.1590/S0100-204X2012001200012
Dean DH (1984) Biochemical genetics of the bacterial insect-control agent Bacillus thuringiensis: basic principles and prospect for genetic engineering. Biotechnol Genet Eng 2:341–363. doi:10.1080/02648725.1984.10647804
Dinardo-Miranda LL (2008) Pragas. In: Dinardo-Mirando LL, Vasconcelos ACM, Landell MGA (eds) Cana-de-açúcar. Instituto Agronômico, Campinas, pp 349–404
Dinardo-Miranda LL, Anjos IA, Costa VP, Fracasso JV (2012) Resistance of sugarcane cultivars to Diatraea saccharalis. Pesq Agropec Bras 47:1–7. doi:10.1590/S0100-204X2012000100001
Dong Z, Canny MJ, McCully ME, Roboredo MG, Cabadilla CF, Ortega E, Rodés R (1994) A nitrogen-fixing endophyte of sugarcane stems (A new role for the apoplast). Plant Physiol 105:1139–1147. doi:10.1104/pp.105.4.1139
Downing KJ, Leslie G, Thomson J (2000) Biocontrol of the sugarcane borer Eldana saccharina by expression of the Bacillus thuringiensis cry1Ac7 and Serratia marcescens chiA genes in sugarcane-associated bacteria. Appl Environ Microbiol 66:2804–2810. doi:10.1128/AEM.66.7.2804- 2810.2000
Estruch JJ, Carozzi NB, Desai N, Duck NB, Warren GW, Koziel MG (1997) Transgenic plants: an emerging approach to pest control. Nat Biotechnol 15:137–141. doi:10.1038/nbt0297-137
Fahey JW, Dimock MB, Tomasino SF, Taylor JM, Carlson PS (1991) Genetically engineered endophytes as biocontrol agents: A case study in industry. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Springer, London, pp 401–411
Falcão-Salles J, Demedeiros-Gitahy P, Skot L, Baldani JL (2000) Use of endophytic diazotrophic bacteria as a vector to express the cry3A gene from Bacillus thuringiensis. Braz J Microbiol 31:155–161. doi:10.1590/S1517-83822000000300001
Falco MC, Silva-Filho MC (2003) Expression of soybean proteinase inhibitors in transgenic sugarcane plants: effects on natural defense against Diatraea saccharalis. Plant Physiol Bioch 41:761–766. doi:10.1016/S0981-9428(03)00100-1
FAOSTAT (2012) Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
Fearing PL, Brown D, Vlachos D, Meghji M, Privalle L (1997) Quantitative analysis of CryIA(b) expression in Bt maize plants, tissue and silage, and stability of expression over successive generations. Mol Breeding 3:169–176. doi:10.1023/A:1009611613475
Ferreira A, Quecine MC, Lacava PT, Oda S, Azevedo JL, Araújo WL (2008) Diversity of endophytic bacteria from Eucalyptus species seeds and colonization of seedlings by Pantoea agglomerans. FEMS Microbiol Lett 287:8–14. doi:10.1111/j.1574-6968.2008.01258.x
Ge AZ, Pwster RM, Dean DH (1990) Hyperexpression of a Bacillus thuringiensis delta-endotoxin-encoding gene in Echerichia coli: properties of the product. Gene 93:49–54. doi:10.1016/0378-1119(90)90134-D
Jeun YC, Park KS, Kim CH, Fowler WD, Kloepper JW (2004) Cytological observation of cucumber plants during induced resistance elicited by rhizobacteria. Biol Control 29:39–42. doi:10.1016/S1049-9644(03)00082-3
King EG, Hartley GG (1985) Diatraea saccharalis. In: Singh HP, Moore RF (eds) Handbook of insects rearing. Elsevier, New York, pp 265–270
Kotze AC, O’Grady J, Gough JM, Pearson R, Bagnall NH, Kemp DH, Akhurst RJ (2005) Toxicity of Bacillus thuringiensis to parasitic and free-living life-stages of nematode parasites of livestock. Int J Parasitol 35:1013–1022. doi:10.1016/j.ijpara.2005.03.010
Liu L, Kloepper JW, Tuzun S (1995) Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology 85:695–698. doi:10.1094/Phyto-85-695
Loiret FG, Ortega E, Kleiner D, Ortega-Rode P, Rode’s R, Dong Z (2004) A putative new endophytic nitrogen-fixing bacterium Pantoea sp. from sugarcane. J Appl Microbiol 97:504–511. doi:10.1111/j.1365-2672.2004.02329.x
Matsuoka S, Garcia AAF, Calheiros GC (1999) Hibridação em cana de- açúcar. In: Borém A (ed) Hibridação Artificial de Plantas. Viçosa, Editora UFV, pp 221–254
Moar WJ, Trumble J, Hice R, Backmann P (1994) Insecticidal activity of the CryIIA protein from the NRD-12 isolate of Bacillus thuringiensis subsp. kurstaki expressed in Escherichia coli and Bacillus thuringiensis and in a leaf-colonizing strain of Bacillus cereus. Appl Environ Microbiol 60:896–902. doi:0099-2240/94/$04.00+0
Nunez WJ, Colmer AR (1968) Differentiation of Aerobacter-Klebsiella isolated from sugarcane. Appl Microbiol 16:1875–1878
Ongena M, Daayf F, Jacques P, Thonart P, Benhamou N, Paulitz TC, Belanger RR (2000) Systemic induction of phytoalexins in cucumber in response to treatments with fluorescent pseudomonads. Plant Pathol 49:523–530. doi:10.1046/j.1365-3059.2000.00468.x
Parra JRP (1996) Técnicas de criação de insetos para programas de controle biológico, 3rd edn. FEALQ, Piracicaba
Parra JRP, Botelho PSM, Pinto AFD (2010) Controle biológico de pragas como um componente chave para a produção sustentável de cana-de-açúcar. In: Barboza-Cortez LA (ed) Bioetanol de cana-de-açucar: P&D para sustentabilidade e produtividade. Blucher, São Paulo, pp 441–450
Perlak FJ, Fuchs RL, Dean DA, Mcpherson S, Fischhoff DA (1991) Modification of the coding sequence enhances plant expression of insect control protein genes. P Natl Acad Sci USA 88:3324–3328. doi:10.1073/pnas.88.8.3324
Pigott CR, Ellar DJ (2007) Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol R 71:255–281. doi:10.1128/MMBR.00034-06
Procópio REL, Araújo WL, Maccheroni-Jr W, Azevedo JL (2009) Characterization of an endophytic bacterial community associated with Eucalyptus spp. Genet Mol Res 8:1408–1422. doi:10.4238/vol8-4gmr691
Quecine MC, Araújo WL, Rossetto PB, Ferreira A, Tsui S, Lacava PT, Mondin M, Azevedo JL, Pizzirani-Kleiner AA (2012) Sugarcane growth promotion by the endophytic bacterium Pantoea agglomerans 33.1. Appl Environ Microbiol 78:7511–7518. doi:10.1128/AEM.00836-12
Ragev A, Keller M, Strizhov N, Sneh B, Prudovsky E, Chet I, Ginzberg I, Koncz-Kalman Z, Koncz C, Schell J, Zilberstein A (1996) Synergistic activity of a Bacillus thuringiensis δ-endotoxin and a bacterial endochitinase against Spodoptera littoralis. Appl Environ Microbiol 62:3581–3586
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Theoduloz C, Vega A, Salazar M, Gonzalez E, Meza-Basso L (2003) Expression of a Bacillus thuringiensis delta-endotoxin cry1Ab gene in Bacillus subtilis and Bacillus licheniformis strains that naturally colonize the phylloplane of tomato plants (Lycopersicon esculentum, Mills). J Appl Microbiol 94:375–381. doi:10.1046/j.1365-2672.2003.01840.x
Tomasino SF, Leister RT, Dimock MB, Beach RM, Kelly JL (1995) Field performance of Clavibacter xyli subsp. cynodontis expressing the insecticidal protein gene cryIA (c) of Bacillus thuringiensis against European corn borer in field corn. Biol Control 5:442–448. doi:10.1006/bcon.1995.1053
Torres AR, Araújo WL, Cursino L, Rossetto PB, Mondin M, Hungria M, Azevedo JL (2013) Colonization of Madagascar periwinkle (Catharanthus roseus), by endophytes encoding gfp marker. Arch Microbiol 195:483–489. doi:10.1007/s00203-013-0897-3
Turner JT, Lampel JS, Stearman RS, Sundin GW, Gunyuzlu P, Anderson JJ (1991) Stability of the delta-endotoxin gene from Bacillus thuringiensis subsp. kurstaki in a recombinant strain of Clavibacter xily subsp. cyondontis. Appl Environ Microbiol 57:3522–3528
Udayasurian V, Nakamura A, Masaki H, Uozumi T (1995) Transfer of an insecticidal protein gene of Bacillus thuringiensis into plant-colonizing Azospirillum. World J Microbiol Biotechnol 11:163–167. doi:10.1007/BF00704640
Veiga ACP, Vacari AM, Volpe HXL, Laurentis VL, De Bortoli SA (2013) Quality control of Cotesia flavipes (Cameron) (Hymenoptera: Braconidae) from different Brazilian bio-factories. Biocontrol Sci Technol 23:665–673. doi:10.1080/09583157.2013.790932
Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV (2003) Bacillus thuringiensis crystal proteins that target nematodes. P Natl Acad Sci USA 100:2760–2765. doi:10.1073/pnas.0538072100
Xue J, Liang G, Crickmore N, Li H, He K, Song F, Feng X, Huang D, Zhang J (2008) Cloning and characterization of a novel Cry1A toxin from Bacillus thuringiensis with high toxicity to the Asian corn borer and other lepidopteran insects. FEMS Microbiol Lett 280:95–101. doi:10.1111/j.1574-6968.2007.01053.x
Zhang L, Huang F, Rogers Leonard B, Chen M, Clark T, Zhu YC, Wangila DS, Yang F, Niu Y (2013) Susceptibility of Cry1Ab maize-resistant and -susceptible strains of sugarcane borer (Lepidoptera: Crambidae) to four individual Cry proteins. J Invertebr Pathol 112:267–272. doi:10.1016/j.jip.2012.12.007
Acknowledgments
This work was supported by grants from the São Paulo Research Foundation (FAPESP) (Proc. No. 05-53748-6 and 02/14143-2) and The National Council for Scientific and Technological Development (CNPq). We also thank Neide Graciano Zério for collaborating on the in vitro bioassays and Dr. Sabrina Moutinho Chabregas from CTC (Piracicaba, SP, Brazil) for discussions and for supplying the sugarcane plants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Quecine, M.C., Araújo, W.L., Tsui, S. et al. Control of Diatraea saccharalis by the endophytic Pantoea agglomerans 33.1 expressing cry1Ac7 . Arch Microbiol 196, 227–234 (2014). https://doi.org/10.1007/s00203-014-0962-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-0962-6