Abstract
Summary
Compared to high-impact exercises, moderate-magnitude impacts from odd-loading directions have similar ability to thicken vulnerable cortical regions of the femoral neck. Since odd-impact exercises are mechanically less demanding to the body, this type of exercise can provide a reasonable basis for devising feasible, targeted bone training against hip fragility.
Introduction
Regional cortical thinning at the femoral neck is associated with hip fragility. Here, we investigated whether exercises involving high-magnitude impacts, moderate-magnitude impacts from odd directions, high-magnitude muscle forces, low-magnitude impacts at high repetition rate, or non-impact muscle forces at high repetition rate were associated with thicker femoral neck cortex.
Methods
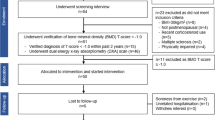
Using three-dimensional magnetic resonance imaging, we scanned the proximal femur of 91 female athletes, representing the above-mentioned five exercise-loadings, and 20 referents. Cortical thickness at the inferior, anterior, superior, and posterior regions of the femoral neck was evaluated. Between-group differences were analyzed with ANCOVA.
Results
For the inferior cortical thickness, only the high-impact group differed significantly (~60%, p = 0.012) from the reference group, while for the anterior cortex, both the high-impact and odd-impact groups differed (~20%, p = 0.042 and p = 0.044, respectively). Also, the posterior cortex was ~20% thicker (p = 0.014 and p = 0.006, respectively) in these two groups.
Conclusions
Odd-impact exercise-loading was associated, similar to high-impact exercise-loading, with ~20% thicker cortex around the femoral neck. Since odd-impact exercises are mechanically less demanding to the body than high-impact exercises, it is argued that this type of bone training would offer a feasible basis for targeted exercise-based prevention of hip fragility.



Similar content being viewed by others
References
Lovejoy CO (1988) Evolution of human walking. Sci Am 259:118–125
Bramble DM, Lieberman DE (2004) Endurance running and the evolution of Homo. Nature 432:345–352
Currey JD (2003) How well are bones designed to resist fracture? J Bone Miner Res 18:591–598
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec 275A:1081–1101
Ruff C, Holt B, Trinkaus E (2006) Who’s afraid of the big bad Wolff?: ‘‘Wolff’s law’’ and bone functional adaptation. Am J Phys Anthropol 129:484–498
Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J (2005) Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet 366:129–135
Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J (1999) Regional differences in cortical porosity in the fractured femoral neck. Bone 24:57–64
Bell KL, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M, Meggitt BF, Reeve J (1999) Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res 14:111–119
Crabtree N, Loveridge N, Parker M, Rushton N, Power J, Bell KL, Beck TJ, Reeve J (2001) Intracapsular hip fracture and the region specific loss of cortical bone: analysis by peripheral quantitative computed tomography. J Bone Miner Res 16:1318–1328
Mayhew P, Kaptoge S, Loveridge N, Power J, Kroger HP, Parker M, Reeve J (2004) Discrimination between cases of hip fracture and controls is improved by hip structural analysis compared to areal bone mineral density. An ex vivo study of the femoral neck. Bone 34:352–361
Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J (2005) Prevention of falls and consequent injuries in elderly people. Lancet 366:1885–1893
Sievänen H, Kannus P (2007) Physical activity reduces the risk of fragility fracture. PLoS Med 4:e222
Järvinen TL, Sievänen H, Khan KM, Heinonen A, Kannus P (2008) Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 336:124–126
Nikander R, Sievänen H, Heinonen A, Karstila T, Kannus P (2008) Load-specific differences in the structure of femoral neck and tibia between world-class moguls skiers and slalom skiers. Scand J Med Sci Sports 8:145–153
Nikander R, Sievanen H, Heinonen A, Kannus P (2005) Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res 20:520–528
Heinonen A, Sievanen H, Kyrolainen H, Perttunen J, Kannus P (2001) Mineral mass, size, and estimated mechanical strength of triple jumpers’ lower limb. Bone 29:279–285
Bolotin HH, Sievanen H, Grashuis JL, Kuiper JW, Jarvinen TL (2001) Inaccuracies inherent in patient-specific dual-energy X-ray absorptiometry bone mineral density measurements: comprehensive phantom-based evaluation. J Bone Miner Res 6:417–426
Beck TJ (2007) Extending DXA beyond bone mineral density: understanding hip structural analysis. Curr Osteoporos Rep 5:49–55
McKay HA, Sievanen H, Petit MA, MacKelvie K, Forkheim KA, Whittall KP, Forster BB, MacDonald H (2004) Application of magnetic resonance imaging to evaluation of femoral neck structure in growing girls. J Clin Densitom 7:161–168
Gomberg BR, Saha PK, Wehrli FW (2005) Method for cortical bone structural analysis from magnetic resonance images. Acad Radiol 12:1320–1332
Sievanen H, Karstila T, Apuli P, Kannus P (2007) Magnetic resonance imaging of the femoral neck cortex. Acta Radiol 48:308–314
Nikander R, Sievänen H, Heinonen A, Kannus P (2006) Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity—a pQCT study of adult female athletes. Bone 39:886–894
Heinonen A, Sievanen H, Viitasalo J, Pasanen M, Oja P, Vuori I (1994) Reproducibility of computer measurement of maximal isometric strength and electromyography in sedentary middle-aged women. Eur J Appl Physiol 68:310–314
Torvinen S, Sievanen H, Jarvinen TA, Pasanen M, Kontulainen S, Kannus P (2002) Effect of 4-min vertical whole body vibration on muscle performance and body balance: a randomized cross-over study. Int J Sports Med 23:374–379
Sievänen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I (1998) Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J Bone Miner Res 13:871–882
Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I (2002) Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 17:2281–2289
Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I (2002) Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int 70:469–474
Parkkari J, Kannus P, Palvanen M, Natri A, Vainio J, Aho H, Vuori I, Järvinen M (1999) Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int 65:183–187
Feskanich D, Willett W, Colditz G (2002) Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA 288:2300–2306
Michaelsson K, Olofsson H, Jensevik K, Larsson S, Mallmin H, Berglund L, Vessby M, Melhus H (2007) Leisure physical activity and the risk of fracture in men. PLoS Med 4:e199
Nordström A, Karlsson C, Nyquist F, Olsson T, Nordström P, Karlsson M (2005) Bone loss and fracture risk after reduced physical activity. J Bone Miner Res 20:202–207
Lotz JC, Cheal EJ, Hayes WC (1995) Stress distributions within the proximal femur during gait and falls: implications for osteoporotic fracture. Osteoporos Int 5:252–261
Hayes WC, Myers ER, Robinovich SN, van der Kroonenberg A, Courtney AC, McMahon TA (1996) Etiology and prevention of age-related hip-fractures. Bone 18:77S–86S
Vainionpaa A, Korpelainen R, Vihriala E, Rinta-Paavola A, Leppaluoto J, Jamsa T (2006) Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int 17:455–463
van den Bogert AJ, Read L, Nigg BM (1999) An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc 31:131–142
Daly RM, Rich PA, Klein R, Bass S (1999) Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: a prospective study in young male gymnasts. J Bone Miner Res 14:1222–1230
Heinonen A, Kannus P, Sievänen H, Oja P, Pasanen M, Rinne M, Uusi–Rasi K, Vuori I (1996) Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 348:1343–1347
Uusi-Rasi K, Kannus P, Cheng S, Sievänen H, Pasanen M, Heinonen A, Nenonen A, Halleen J, Fuerst T, Genant H, Vuori I (2003) Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone 33:132–143
Bergström I, Landgren BM, Brinck J, Freyschuss B (2008) Physical training preserves bone mineral density in postmenopausal women with forearm fractures and low bone mineral density. Osteopor Int 19:177–188
Karinkanta S, Heinonen A, Sievänen HA, Uusi-Rasi K, Pasanen M, Ojala K, Fogelholm M, Kannus P (2007) Multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 18:453–462
Liu-Ambrose TY, Khan KM, Eng JJ, Heinonen A, McKay HA (2004) Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Dens 7:390–398
Korpelainen R, Keinänen-Kiukaanniemi S, Heikkinen J, Väänänen K, Korpelainen J (2006) Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos Int 17:109–118
Acknowledgments
We thank all the participants for their contribution and time, including Saturdays, for this study. The collaboration with the involved sports associations and sports clubs as well as with their coaches is greatly appreciated. We thank Ms Taru Helenius for managing the measurement schedule, Ms Ulla Hakala and Ms Ulla Honkanen for DXA and pQCT measurements at the UKK Institute, and Ms Arja Hilander and Ms Anu Kuhanen for the MRI measurements at the Tampere University Hospital. The help of Chief Physicist Pertti Ryymin from the Tampere University Hospital in defining the appropriate MRI sequence for this study is gratefully acknowledged. The financial support of the Medical Fund of the Pirkanmaa Hospital District, Finnish Ministry of Education, National Graduate School for Musculoskeletal Disorders and Biomaterials, and the Päivikki and Sakari Sohlberg Foundation is greatly appreciated.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikander, R., Kannus, P., Dastidar, P. et al. Targeted exercises against hip fragility. Osteoporos Int 20, 1321–1328 (2009). https://doi.org/10.1007/s00198-008-0785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0785-x