Abstract
Purpose
Patients requiring mechanical ventilation (MV) for >48 h after major heart surgery (MHS) are at a high risk of acquiring ventilator-associated pneumonia (VAP) and tracheobronchitis (VAT). Most non-pharmacological interventions to prevent VAP in such patients are usually already implemented. The objective of this study was to evaluate the efficacy in preventing lower respiratory infections of antibiotics active against multidrug-resistant pathogens in this very high-risk population.
Methods
We performed a prospective randomized open-label study of MHS patients requiring MV for >48 h. Patients were randomly allocated to one of two groups: the intervention group, which received a 3-day course of linezolid and meropenem, and the control group, which received the standard of care. The main outcome was the development of VAP or VAT.
Results
Overall, of the 78 patients included in the study, 40 were in the intervention group and 38 in the control group. Both groups were comparable. Data for the intervention and control groups respectively were as follows: VAP + VAT/1,000 days was 31.79 vs 64.78 (p = 0.03), median length of MV before the first episode of VAP or VAT 9 vs 4.5 days (p = 0.02). No significant differences were observed in median length of stay in the intensive care unit, median length of hospital stay, antibiotic use, Clostridium difficile infection, and overall mortality rate. We detected linezolid-resistant coagulase-negative and coagulase-positive staphylococci in the MHS intensive care unit after the study period.
Conclusions
A pre-emptive approach with broad-spectrum antibiotics may be effective in reducing the incidence and delaying the onset of VAP + VAT after MHS. The ecological consequences have to be carefully evaluated in future trials.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is the most frequent infection in patients admitted to intensive care units (ICUs) and is associated with prolonged hospitalization [1–3], increased health-care costs [4], and a 15–45 % attributable mortality rate in most studies [5–7].
Patients undergoing major heart surgery (MHS) are usually elderly and have many underlying conditions predisposing to VAP, which is the most common postoperative infection in this population [2, 8–12]. In particular, patients remaining under mechanical ventilation (MV) for >48 h after MHS have a very high risk of VAP (46 % of cases in a recent study) [9].
The number of variables in this high-risk group that are amenable to intervention with conventional non-pharmacologic preventive measures is very limited. To our knowledge, the value and consequences of pre-emptive therapy with broad-spectrum antibiotics in the prevention of VAP have not been assessed in randomized trials.
Our study attempted to determine whether a short course of broad-spectrum antimicrobial agents could reduce the incidence or delay the onset of lower respiratory tract infections (LRT) in this high-risk population.
Materials and methods
Our institution is a general reference hospital with 1,550 beds and approximately 50,000 admissions/year. The Department of Cardiovascular Surgery is a large referral unit that performs more than 500 MHS procedures annually. The MHS unit has 14 beds, with a median occupancy rate of 80–90 %. The usual prevention measures for VAP include semirecumbent position of intubated patients, oral hygiene, oral intubation, and early weaning. No intestinal decontamination is used.
Study design
This was a prospective randomized open-label academic clinical trial (registration number PI070896) that included patients who fulfilled all the following criteria: recent MHS and MV of >48 h, age greater than 18 years, no evidence or suspicion of infection at enrollment, not receiving antibiotics other than those prescribed (within 24 h) for surgical prophylaxis, no history of allergy or intolerance to study drugs, and no pregnancy.
During the study period (January 2007–December 2009), patients who gave their informed consent before surgery were randomly assigned to one of two groups: the intervention group and the control group. Immediately after enrollment, patients in the intervention group received 3 days of pre-emptive therapy (meropenem and linezolid) at standard doses; patients in the control group were treated according to standard practice and received antibiotics only if clinically indicated. Meropenem was administered at 1 g/8 h IV in patients without renal failure; linezolid was administered at 600 mg/12 h IV. In patients unable to receive linezolid for any reason, vancomycin (25 mg/kg IV) adjusted to blood levels was used as an alternative against potential gram-positive microorganisms.
Microbiological methods and sampling
LRT samples were routinely obtained on three consecutive days after enrollment and when clinically indicated. Endotracheal aspirates were obtained daily on the 3 days after inclusion and were quantitatively processed. Specimens with 104 cfu/ml or more of microorganisms that are usually causative of pneumonia were considered significant. This threshold was selected in order to increase sensitivity. Only patients with signs and symptoms attributable to the pathogen were considered infected. Furthermore, on day 1 following enrollment (day +3 after surgery), a protected specimen brush culture was also undertaken and considered significant if counts greater than 103 cfu/ml of significant bacteria were obtained.
Sampling of the lower respiratory tract in cases of suspected VAP, ventilator-associated tracheobronchitis (VAT), or both was performed either by endotracheal aspiration (EA) and/or protected brush. When aspiration was unproductive, we irrigated with 5 ml of Ringer’s lactate solution. Secretions obtained by endotracheal aspiration were collected in a Lukens specimen container (Sherwood Medical, Tullamore, Ireland). A sample was considered positive with bacterial counts at least 104 cfu/ml of each microorganism obtained using EA and at least 103 cfu/ml of each microorganism obtained using protected brushing.
All microorganisms were identified using standard methods, and antimicrobial susceptibility was determined according to Clinical and Laboratory Standards Institute (CLSI) recommendations. Linezolid minimum inhibitory concentrations (MICs) were determined by both the standard microdilution procedures and by E-test [13].
Definition of VAP
Patients ventilated for >48 h were diagnosed with VAP on the basis of the presence of new and/or progressive pulmonary infiltrates on the chest radiograph plus two or more of the following criteria: fever greater than 38.5 °C or hypothermia less than 36 °C, leukocytosis of 12 × 109/L or more, purulent tracheobronchial secretions, or a reduction in PaO2/FiO2 of 15 % or more according to the definitions of the Centers for Disease Control and Prevention (CDC) [14, 15]. The isolation of one or more pathogenic microorganisms in significant bacterial counts was required to confirm the diagnosis of VAP.
The definition for diagnosis of VAT was identical to that of VAP in the absence of pulmonary infiltrates. In order to exclude colonization, patients with tracheobronchitis had to present fever or hypothermia, leukocytosis, purulent tracheobronchial secretions, or a reduction in PaO2/FiO2 of 15 % or more not attributable to other causes [15]. Only the first episode of pneumonia was considered. Patients with pneumonia after a tracheobronchitis episode were counted only as pneumonia.
We considered as nonpathogenic the isolation (at any concentration) of the following microorganisms in LRT secretions: viridans-group Streptococci, coagulase-negative staphylococci, Neissseria spp., Corynebacterium spp., and Candida spp., unless other evidence was available.
Primary endpoint
The main endpoint of the study was the reduction in the incidence and incidence density of VAP, VAT, or the combination of both (LRTI) in the intervention group.
Secondary endpoints
Our secondary endpoints were days of MV, ICU and hospital length of stay, mortality rate, cost of antimicrobial therapy during ICU stay, and ecological impact on the MHS–ICU population (emergence of antimicrobial resistance, superinfection, and Clostridium difficile infection (CDI).
Ethics
The ethics committee of our institution approved the study and all patients gave their informed consent before inclusion in the study.
Follow-up
Patients were followed up daily to check for the presence of infections by physicians from the Department of Anesthesia and infectious disease specialists participating in the study. Clinical data were recorded according to a preestablished protocol, and no further systematic surveillance respiratory tract cultures were performed.
Presurgical information
Presurgical information included epidemiological data, underlying diseases, and standard scores (American Society of Anesthesiologists score, EuroSCORE, Charlson comorbidity index, Canadian score, and APACHE II score) on admission to the ICU and to the study [16].
Surgical information
Surgical information included type of surgery, indication, duration, time on cardiopulmonary bypass, aortic cross-clamp time, transfusion needs, reinterventions, antimicrobial prophylaxis, and need for inotropic support. Antimicrobial prophylaxis for surgery consisted of 2 g of cefazolin given before surgery and every 8 h thereafter for a total of three doses. One patient who was allergic to cefazolin received vancomycin (1 g) before surgery.
Postsurgical outcome
Events included ICU and hospital length of stay, hours on MV, and need for tracheostomy. Infections other than VAP were recorded. In patients with sepsis, the bone score for severity of sepsis was also recorded. Enrolled patients were prospectively followed to monitor occurrence of VAP, VAT, or both until they were successfully weaned from MV, discharged from the hospital, or died. Outcome variables also included antimicrobial administration, C. difficile-associated infection, ICU length of stay, ICU mortality, hospital length of stay, and mortality at discharge.
Ecological impact on the MHS–ICU
In order to assess the ecological impact of antibiotic therapy on the MHS–ICU, we obtained and compared data from the Microbiology Department during two periods, namely, before and after the study. The period of 3 years before the study (included the years 2004–2006 and the period after the study included the years 2008–2010).
Statistical analysis
A sample size of 116 patients, 58 in each arm, was estimated considering that the intervention would cause a difference of 50 % between groups in the incidence of LRTI using a two-tailed z test of proportions between two groups with 80 % power and a 5 % level of significance. The study was interrupted prematurely owing to the emergence of an outbreak of coagulase-negative staphylococci in the unit that was initially interpreted as purportedly related to the study.
Relationships between baseline variables were evaluated for both groups. Baseline comparisons between groups were established by clinical relevance according to the CONSORT recommendations [17]. Qualitative variables appear with their frequency distribution. Quantitative variables are expressed as the mean and standard deviation (SD) and as the median and interquartile range (IQR) if their distribution was skewed. Normally distributed continuous variables were compared using the t test; non-normally distributed continuous variables were compared using the median test. The chi-squared or Fisher exact test was used to compare categorical variables.
The incidence rates of respiratory tract infection (VAP and/or VAT) (event/1,000 days of MV) between the groups were compared using Cox regression analysis including all the variables statistically associated with treatment in the bivariate analysis (age, coronary surgery, and McCabe and Jackson scale which were found to be different in both groups). We calculated the adjusted hazard ratio controlling for these variables, with the purpose of excluding potential confounding in our final conclusions. The hazard ratio (HR) and 95 % confidence interval (CI) were calculated.
All statistical tests were two-tailed. Statistical significance was set at p <0.05 for all the tests. The statistical analysis was performed with SPSS 12.0 and Stata 9.0.
Results
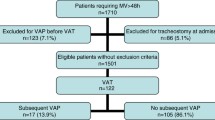
During the study period, 1,250 patients underwent MHS. Of these, 234 had to remain under MV for >48 h. Finally, 156 cases had to be excluded for different reasons, such as receiving antimicrobial therapy other than surgical prophylaxis, refusal to participate in the study, emergency surgery, transplantation, or others. The remaining 78 patients comprised the intention-to-treat population and the per-protocol population and were randomized to the intervention group (40 patients) or the control group (38 patients) (Fig. 1 in the electronic supplementary material).
The underlying conditions and surgical variables of both populations are compared in Table 1 and described in the electronic supplementary material.
Outcome
The outcomes of both groups are summarized in Table 2. Median ventilatory days (IQR) were similar in both groups: 6 (4–17.5) vs 6 (4–16), although, as mortality was higher in the control group, patients in the intervention group were able to be ventilated longer: 444 vs 503 days (not significant, NS). The cumulative incidence density of VAP during the study period was 20.88 episodes/1,000 days of MV. When VAP and VAT were considered separately, we were unable to demonstrate a significant difference in the incidence or incidence density between the two. When episodes of LRTI were analyzed together, we found a significant difference in the incidence density between the intervention and control groups (31.79/1,000 days of ventilation [intervention] vs 64.78 episodes/1,000 days of ventilation [control], p = 0.03).
The episodes of VAP or VAT occurred significantly later in the intervention group (9.0 days [IQR 9–23] vs 4.5 days, [IQR 3–9], p = 0.02). We were not able to demonstrate other differences in the secondary endpoints of the study, including days of ICU and hospital stay, days on MV, and need for tracheostomy. No differences were detected between groups in the incidence of other infections, consumption of antimicrobial agents, or incidence of C. difficile infections in MHS–ICU or hospital mortality (Table 2). Nine of the 15 patients with VAP developed severe sepsis and 6 had positive blood cultures.
Microbiology of colonization and VAP/tracheobronchitis
The microorganisms considered colonizers isolated from the LRT in both groups (one microorganism per patient) are compared in Table 3. No trends towards colonization with different microorganisms were detected between the groups. The microorganisms causing LRTI in both groups are summarized in (Fig. 2 [one microorganism per episode] [see electronic supplementary material]). We were unable to demonstrate any significant differences in the etiologic agents causing VAP or VAT between the groups.
Ecological impact of the implementation of pre-emptive treatment with broad-spectrum antibiotics in the MHS–ICU
In order to assess the ecological impact of the introduction of the pre-emptive approach, we analyzed several parameters in the whole population admitted to the MHS–ICU during a period of 3 years before the introduction of the protocol (2004–2006) and during a 3-year follow-up period beginning at the end of the first year of the protocol (2008–2010) (Table 4).
Table 4 shows data obtained from the Microbiology Department on the evolution of the incidence per 1,000 MHS/ICU admissions of several parameters during both periods. When we analyzed the daily defined doses (DDDs) of meropenem and linezolid prescribed in the whole population of the MHS unit in both periods, only meropenem showed a significant increase (mean DDDs/100 hospital stays 16.7 in the first period and 24.3 in the second one, p = 0.04).
We were unable to demonstrate any significant increase in the number of microorganisms isolated in the Microbiology Department or in the incidence of infections caused by multidrug-resistant gram-negative bacteria. There was no increase in the infections caused by P. aeruginosa, meropenem-resistant P. aeruginosa, or in the episodes of MDR A. baumannii.
Regarding gram-positive infections, there was a very significant decrease in methicillin-resistant (p < 0.01) and methicillin-susceptible S. aureus infections. However, during the post-study period, we detected linezolid-resistant coagulase-negative staphylococci (20 patients) and linezolid-resistant S. aureus (one patient) as colonizers or agents of infection that were not present before the study. All coagulase-negative staphylococci showed a MIC greater than 4 mg/L by both methods (microdilution and E-test). MICs ranged from 8 to greater than 256 mg/L. The mechanisms of linezolid resistance were multiple. Nine coagulase-negative isolates presented the cfr gene, six had the G2576T mutation, and the remaining five presented both mechanisms. The linezolid-resistant S. aureus had the cfr gene. Out of the 21 patients with linezolid R isolates, 13 presented infections and the remaining 8 were colonized.
Multivariate analysis
In a multivariate analysis adjusted for age, coronary bypass surgery, and prognosis of the underlying conditions, the differences in the incidence density of LRT infection between the control and the intervention groups remained significant (Table 5).
Discussion
Our study shows that even a 3-day course of broad-spectrum antimicrobial agents can reduce the incidence and delay the onset of LRTI in high-risk patients undergoing MV after MHS. However, we were unable to demonstrate a reduction in days of ICU stay or mortality between the study groups. Following the study period, we detected an increase in the number of linezolid-resistant Staphylococcus spp. (coagulase-negative staphylococci and S. aureus) in the MHS–ICU.
VAP is the most frequent infection after MHS, with incidence rates ranging from 5.7 to 21.6 % and incidence densities ranging from 22.2/1,000 days of MV to 34.5/1,000 days of MV in all patients undergoing surgery [2, 9, 18–20]. However, patients who need to remain under MV for >48 h after MHS constitute a group with a particularly high risk [8, 18, 19]. In these patients, members of our team previously reported that 46 % of the patients developed VAP [9].
Non-pharmacologic preventive measures are usually applied systematically in this population, and few variables remain amenable to intervention [2, 9, 18, 21, 22].
Regarding the use of antimicrobial agents for prophylaxis or pre-emptive treatment of VAP, strategies such as local oral hygiene with chlorhexidine are still under debate [23, 24]. The use of aerosolized antibiotics has been repeatedly discussed, although no firm conclusions regarding efficacy have been reached [25, 26].
Selective digestive decontamination (SDD) with topical antibiotics (with or without parenteral antibiotics) also remains open to debate [27]. A meta-analysis carried out by Silvestri et al. [28] between 1987 and 2005 analyzed 8,065 critically ill patients and concluded that SDD significantly reduced overall and gram-negative bloodstream infections and mortality. Other studies also reported a decreased incidence of VAP [28–31]. A Cochrane systematic review showed that a combination of topical and systemic prophylactic antibiotics reduced LRTI and overall mortality in adult patients receiving intensive care. Prophylaxis based on the use of topical agents alone reduces respiratory infections but not mortality [32, 33]. The most common systemic antibiotic used for the prevention of VAP is ceftriaxone, usually administered in a short course. However, this approach has an inadequate spectrum and duration to eliminate the most common pathogens causing late VAP, including methicillin-resistant S. aureus, P. aeruginosa, and extended-spectrum β-lactamase-producing Enterobacteriaceae [34]. We speculated that if systemic antibiotics were the most effective component of SDD, perhaps a combination with broader-spectrum agents applied for a period of 3 days could be even more effective [35]. We selected this broad-spectrum regimen because previous data from this same unit had demonstrated that resistant pathogens were also involved in early VAPs [36]. In fact, many of these patients are operated on after several days of hospital stay, thus increasing the risk of MDR pathogens.
Although our data show a reduction in the incidence density of LRTI, we were unable to demonstrate statistical significance for VAP or VAT as individual conditions, probably as a result of the limited number of patients we were able to include in the study.
The second major point of our study is the potential ecological impact of a short but broad-spectrum pre-emptive treatment. Resistance development in SDD has always been a cause of concern, but this risk has never been demonstrated in clinical trials investigating SDD [37, 38].
Indicators and the proper moment to monitor ecological impacts of antibiotic stewardship are far from clear, but, in our opinion, assessment of resistance should be extended to untreated patients in the ICU. Resistance may not develop during the study or immediately after its conclusion, but it could be delayed and appear in other types of patients. To our knowledge, this possibility has not been thoroughly evaluated.
In the ICU where the study was performed, we did not witness significant resistance among the gram-negative bacteria isolated from the included patients. However, we detected linezolid-resistant microorganisms in the MHS–ICU (mainly coagulase-negative staphylococci, but also one case of S. aureus). The causality relationship between the use of linezolid in the protocol and the emergence of resistance cannot be established, because the overall consumption of linezolid did not increase significantly during the study period. The mechanisms of linezolid resistance were varied and involved different clones. One year later (2011), one outbreak of linezolid-resistant S. aureus occurred in another ICU of the hospital [13]. It was caused by a different clone than the isolate from the MHS–ICU, and was rapidly controlled.
The main limitation of our study is the small number of cases enrolled, given that many high-risk patients did not fulfill the criteria for enrollment. The second limitation is that we enrolled only patients at high risk of infection after MHS; consequently, our findings cannot necessarily be extrapolated to other populations. Finally, the study was not blinded and was performed in a single center. However, our aim was to demonstrate this effect as a proof of concept in a very high-risk and uniform population, such as patients who have just undergone MHS.
Our study shows that a relatively short course of broad-spectrum antibiotics can reduce the incidence and delay the onset of LRT infection in high-risk patients after MHS. These results must be balanced with the risk of inducing ecological changes with the presence of multidrug microorganisms. In our opinion, other drugs and/or longer courses of pre-emptive treatment must be assessed.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- CASS:
-
Continuous aspiration of subglottic secretions
- CDI:
-
Clostridium difficile infection
- CDC:
-
Centers for Disease Control and Prevention
- DDDs:
-
Daily defined doses
- EuroSCORE:
-
European System for Cardiac Operative Risk Evaluation
- EA:
-
Endotracheal aspirates
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LRTI:
-
Lower respiratory tract infections
- MHS:
-
Major heart surgery
- MV:
-
Mechanical ventilation
- NYH:
-
New York Heart Association
- SD:
-
Standard deviation
- VAP:
-
Ventilator-associated pneumonia
- VAT:
-
Ventilator-associated tracheobronchitis
References
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH, VAP Outcomes Scientific Advisory Group (2002) Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 122:2115–2121
Bouza E, Hortal J, Munoz P, Pascau J, Perez MJ, Hiesmayr M (2006) Postoperative infections after major heart surgery and prevention of ventilator-associated pneumonia: a one-day European prevalence study (ESGNI-008). J Hosp Infect 64:224–230
Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C (1999) The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med 159:1249–1256
Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ (2003) Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med 31:1312–1317
Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C (1993) Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 94:281–288
Cunnion KM, Weber DJ, Broadhead WE, Hanson LC, Pieper CF, Rutala WA (1996) Risk factors for nosocomial pneumonia: comparing adult critical-care populations. Am J Respir Crit Care Med 153:158–162
Bekaert M, Timsit JF, Vansteelandt S, Depuydt P, Vesin A, Garrouste-Orgeas M, Decruyenaere J, Clec’h C, Azoulay E, Benoit D, Outcomerea Study Group (2011) Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med 184:1133–1139
Hortal J, Muñoz P, Cuerpo G, Litvan H, Rosseel PM, Bouza E (2009) Ventilator-associated pneumonia in patients undergoing major heart surgery: an incidence study in Europe. Crit Care 13:R80
Hortal J, Giannella M, Perez MJ, Barrio JM, Desco M, Bouza E, Muñoz P (2009) Incidence and risk factors for ventilator-associated pneumonia after major heart surgery. Intensive Care Med 35:1518–1525
Bouza E, Perez MJ, Muñoz P, Rincon C, Barrio JM, Hortal J (2008) Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest 134:938–946
Sodano L, Agodi A, Barchitta M, Musumeci F, Menichetti A, Bellocchi P, Cunsolo R, Coco G (2004) Nosocomial infections in heart surgery patients: active surveillance in two Italian hospitals. Ann Ig 16:735–743
Leal Noval SR, Marquez VJA, García Curiel A, Camacho Laraña P, Rincón Ferrari MD, Ordoñez Fernández A et al (2000) Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med 28:935–940
Padilla B, Bunsow E, Cercenado E, Marín M, Vicente T, Cantero M, Frías I, Bouza E (2011) Eradication of an outbreak of linezolid-resistant methicillin-resistant Staphylococcus aureus (LR–MRSA) in a post-surgical intensive care unit. In: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, American Society for Microbiology, Chicago, USA
Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R (2004) Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 53(RR-3):1–36
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Campeau L (2002) The Canadian Cardiovascular Society grading of angina pectoris revisited 30 years later. Can J Cardiol 18:371–379
Weller C, McNeil J (2010) CONSORT 2010 statement: updated guidelines can improve wound care. J Wound Care 19:347–353
Kollef MH (1993) Ventilator-associated pneumonia. A multivariate analysis. JAMA 270(16):1965–1970
Bouza E, Hortal J, Muñoz P, Perez MJ, Riesgo MJ, Hiesmayr M (2006) Infections following major heart surgery in European intensive care units: there is room for improvement (ESGNI 007 Study). J Hosp Infect 63:399–405
Craven DE, Kunches LM, Kilinsky V, Lichtenberg DA, Make BJ, McCabe WR (1986) Risk factors for pneumonia and fatality in patients receiving continuous mechanical ventilation. Am Rev Respir Dis 133:792–796
Rello J, Afonso E, Lisboa T, Ricart M, Balsera B, Rovira A, Valles J, Diaz E, FADO Project Investigators (2013) A care bundle approach for prevention of ventilator-associated pneumonia. Clin Microbiol Infect 19:363–369. doi:10.1111/j.1469-0691.2012.03808
Ashraf M, Ostrosky-Zeichner L (2012) Ventilator-associated pneumonia: a review. Hosp Pract (Minneap) 40:93–105
Chlebicki MP, Safdar N (2007) Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med 35:595–602
Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI (2011) Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 11:845–854
Wood GC, Boucher BA, Croce MA, Hanes SD, Herring VL, Fabian TC (2002) Aerosolized ceftazidime for prevention of ventilator-associated pneumonia and drug effects on the proinflammatory response in critically ill trauma patients. Pharmacotherapy 22:972–982
Lode H, Hoffken G, Kemmerich B, Schaberg T (1992) Systemic and endotracheal antibiotic prophylaxis of nosocomial pneumonia in ICU. Intensive Care Med 18(Suppl 1):S24–S27
Vincent JL, Jacobs F (2011) Effect of selective decontamination on antibiotic resistance. Lancet Infect Dis 11:337–338
Silvestri L, van Saene HK, Thomann C, Peric M (2007) Selective decontamination of the digestive tract reduces pneumonia and mortality without resistance emerging. Am J Infect Control 35:354–357
Silvestri L, van Saene HK, Sarginson RE, Gullo A (2007) Selective decontamination of the digestive tract and ventilator-associated pneumonia: we cannot let misinformation go uncorrected. J Intensive Care Med 22:181–182
Taylor N, van Saene HK, Abella A, Silvestri L, Vucic M, Peric M (2007) Selective digestive decontamination. Why don’t we apply the evidence in the clinical practice? Med Intensiva 31:136–145
de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS et al (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev (4): CD000022. doi: 10.1002/14651858.CD000022.pub3
Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M (2011) Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care 15:R155
Sanchez M, Cambronero JA, Lopez J, Cerda E, Rubio J, Gomez MA, Núnez A, Rogero S, Onoro JJ, Sacristán del Castillo JA (1998) Effectiveness and cost of selective decontamination of the digestive tract in critically ill intubated patients. A randomized, double-blind, placebo-controlled, multicenter trial. Am J Respir Crit Care Med 158:908–916
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL (2000) Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162:505–511
Bouza E, Perez A, Muñoz P, Jesus Perez M, Rincon C, Sanchez C, Martín-Rabadán P, Riesgo M, Cardiovascular Infection Study Group (2003) Ventilator-associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med 31:1964–1970
van Essen EH, de Jonge E (2011) Selective decontamination of the digestive tract (SDD): is the game worth the candle? Semin Respir Crit Care Med 32:236–242
Falagas ME, Siempos II, Bliziotis IA, Michalopoulos A (2006) Administration of antibiotics via the respiratory tract for the prevention of ICU-acquired pneumonia: a meta-analysis of comparative trials. Crit Care 10:R123
Mc Cabe WJG (1962) Gram-negative bacteremia, I: etiology and ecology. Arch Inter Med 110:847–855
Charlson M, Pompei P, Ales K, MacKenzie C (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Hurst JW, Morris DC, Alexander RW (1999) The use of the New York Heart Association’s classification of cardiovascular disease as part of the patient’s complete problem list. Clin Cardiol 22:385–390
Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R (1999) European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 16:9–13
Acknowledgments
We thank Thomas O’Boyle for his help in the preparation of the English version of the manuscript and Cristina Fernández for the statistical analysis. Supported in part by Ciber de Enfermedades Respiratorias (CIBERES) and by the Rafael del Pino Foundation. This study was partially supported by grants from the Fondo de Investigación Sanitaria FIS PI070896, FIS PIO9/1257, and FISPI10/02869 (Instituto de Salud Carlos III).
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at doi:10.1007/s00134-013-2983-z.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bouza, E., Granda, M.J.P., Hortal, J. et al. Pre-emptive broad-spectrum treatment for ventilator-associated pneumonia in high-risk patients. Intensive Care Med 39, 1547–1555 (2013). https://doi.org/10.1007/s00134-013-2997-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2997-6