Abstract
Aims/hypothesis
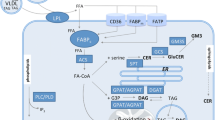
Insulin-sensitive tissues (muscle, liver) of individuals with obesity and type 2 diabetes mellitus are in a state of low-grade inflammation, characterised by increased Toll-like receptor (TLR) expression and TLR-driven signalling. However, the cause of this mild inflammatory state is unclear. We tested the hypothesis that a prolonged mild increase in plasma NEFA will increase TLR expression and TLR-driven signalling (nuclear factor κB [NFκB] and mitogen-activated kinase [MAPK]) and impair insulin action in muscle of lean healthy individuals.
Methods
Twelve lean, normal-glucose-tolerant participants were randomised to receive a 48 h infusion (30 ml/h) of saline or Intralipid followed by a euglycaemic–hyperinsulinaemic clamp. Vastus lateralis muscle biopsies were performed before and during the clamp.
Results
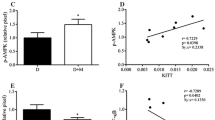
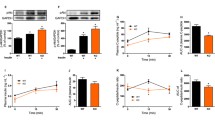
Lipid infusion impaired insulin-stimulated IRS-1 tyrosine phosphorylation and reduced peripheral insulin sensitivity (p < 0.01). The elevation in circulating NEFA increased expression of TLR3, TLR4 and TLR5, and several MAPK (MAPK8, MAP4K4, MAP2K3) and inhibitor of κB kinase-NFκB (CHUK [IKKA], c-REL [REL] and p65 [RELA, NFKB3, p65]) signalling genes (p < 0.05). The lipid infusion also increased extracellular signal-regulated kinase (ERK) phosphorylation (p < 0.05) and tended to reduce the content of inhibitor of kappa Bα (p = 0.09). The muscle content of most diacylglycerol, ceramide and acylcarnitine species was unaffected. In summary, insulin resistance induced by prolonged low-dose lipid infusion occurs together with increased TLR-driven inflammatory signalling and impaired insulin-stimulated IRS-1 tyrosine phosphorylation.
Conclusions/interpretation
A sustained, mild elevation in plasma NEFA is sufficient to increase TLR expression and TLR-driven signalling (NFκB and MAPK) in lean individuals. The activation of this pathway by NEFA may be involved in the pathogenesis of insulin resistance in humans.
Trial registration ClinicalTrials.gov NCT01740817






Similar content being viewed by others
Abbreviations
- DAG:
-
Diacylglycerol
- ERK:
-
Extracellular signal-regulated kinase
- ESI-MS/MS:
-
Electrospray tandem mass spectrometry
- GSK-3:
-
Glycogen synthase kinase-3
- HOMA-IR:
-
HOMA of insulin resistance
- IκBα:
-
Inhibitor of kappa Bα
- IKK:
-
Inhibitor of κB kinase
- JNK:
-
c-Jun N-terminal kinase
- LC-MS/MS:
-
High-performance liquid chromatography/mass spectrometry
- M :
-
Insulin-stimulated glucose metabolism
- MAPK:
-
Mitogen-activated kinase
- NFκB:
-
Nuclear factor κB
- PI3K:
-
Phosphatidylinositol 3-kinase
- TLR:
-
Toll-like receptor
References
Miles JM, Wooldridge D, Grellner WJ et al (2003) Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 52:675–681
Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA (2004) Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab 89:4649–4655
Boden G, Chen X, Rosner J, Barton M (1995) Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44:1239–1242
Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51:2005–2011
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025
Senn JJ (2006) Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281:26865–26875
Schenk S, Saberi M, Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118:2992–3002
Holland WL, Bikman BT, Wang LP et al (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121:1858–1870
Reyna SM, Ghosh S, Tantiwong P et al (2008) Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57:2595–2602
Bouzakri K, Roques M, Gual P et al (2003) Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52:1319–1325
Koistinen HA, Chibalin AV, Zierath JR (2003) Aberrant p38 mitogen-activated protein kinase signalling in skeletal muscle from type 2 diabetic patients. Diabetologia 46:1324–1328
Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM (2005) Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 54:2351–2359
Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D (2007) Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 92:3967–3972
Bachmann OP, Dahl DB, Brechtel K et al (2001) Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50:2579–2584
Hoeg LD, Sjoberg KA, Jeppesen J et al (2011) Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes 60:64–73
Vistisen B, Hellgren LI, Vadset T et al (2008) Effect of gender on lipid-induced insulin resistance in obese subjects. Eur J Endocrinol 158:61–68
Serlie MJ, Allick G, Groener JE et al (2007) Chronic treatment with pioglitazone does not protect obese patients with diabetes mellitus type II from free fatty acid-induced insulin resistance. J Clin Endocrinol Metab 92:166–171
Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM (2002) Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab 87:226–234
Dresner A, Laurent D, Marcucci M et al (1999) Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259
Belfort R, Mandarino L, Kashyap S et al (2005) Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54:1640–1648
Radin MS, Sinha S, Bhatt BA, Dedousis N, O’Doherty RM (2008) Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia 51:336–346
Hussey SE, Liang H, Costford SR et al (2012) TAK-242, a small molecule inhibitor of toll-like receptor-4 signaling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci Rep 33:37–47
Sinha S, Perdomo G, Brown NF, O’Doherty RM (2004) Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279:41294–41301
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Tantiwong P, Shanmugasundaram K, Monroy A et al (2010) NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab 299:E794–E801
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP (1981) The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30:1000–1007
Dube JJ, Bhatt BA, Dedousis N, Bonen A, O’Doherty RM (2007) Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol 293:R642–R650
Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv Exp Med Biol 688:46–59
Gao Z, Hwang D, Bataille F et al (2002) Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277:48115–48121
Hoy AJ, Brandon AE, Turner N et al (2009) Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol Endocrinol Metab 297:E67–E75
Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ (2009) Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94:3543–3549
Kashyap S, Belfort R, Gastaldelli A et al (2003) A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52:2461–2474
Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB (1999) Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest 104:733–741
Inoue G, Cheatham B, Emkey R, Kahn CR (1998) Dynamics of insulin signaling in 3T3-L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J Biol Chem 273:11548–11555
Bhatt BA, Dube JJ, Dedousis N, Reider JA, O’Doherty RM (2006) Diet-induced obesity and acute hyperlipidemia reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol 290:R233–R240
Tripathy D, Mohanty P, Dhindsa S et al (2003) Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887
Lee JY, Plakidas A, Lee WH et al (2003) Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 44:479–486
Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS (2004) Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 382:619–629
Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L (2012) Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55:1140–1150
Adams SH, Hoppel CL, Lok KH et al (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139:1073–1081
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62:1–8
Pal D, Dasgupta S, Kundu R et al (2012) Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 18:1279–1285
Dasgupta S, Bhattacharya S, Biswas A, Majumdar SS, Mukhopadhyay S, Ray S (2010) NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochem J 429:451–462
Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO (2013) Associations of serum fetuin-A levels with insulin resistance and vascular complications in patients with type 2 diabetes. Diab Vasc Dis Res 10:459–467
Song MJ, Kim KH, Yoon JM, Kim JB (2006) Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 346:739–745
Ladefoged M, Buschard K, Hansen AM (2012) Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS 121:531–538
Ahmad R, Al-Mass A, Atizado V et al (2012) Elevated expression of the toll like receptors 2 and 4 in obese individuals: its significance for obesity-induced inflammation. J Inflamm (Lond) 9:48
Dasu MR, Devaraj S, Park S, Jialal I (2010) Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33:861–868
Creely SJ, McTernan PG, Kusminski CM et al (2007) Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292:E740–E747
Wu LH, Huang CC, Adhikarakunnathu S et al (2012) Loss of toll-like receptor 3 function improves glucose tolerance and reduces liver steatosis in obese mice. Metabolism 61:1633–1645
Acknowledgements
We thank all the study participants and their families for their time and participation.
Funding
This work was supported by grants from the National Institutes of Health (NIH) (RO1-DK80157 and RO1-DK089229) and the American Diabetes Association to NM. This grant was supported by a University of Texas Health Science Center at San Antonio Clinical and Translational Science Award (TR000149) and an NIH National Service Research Award, Parent F32 (1F32DK095565-01A1) to SH. The research was supported in part by the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (MUSC) (P30 CA138313) and the Lipidomics Core in the South Carolina Lipidomics and Pathobiology COBRE, Department Biochemistry, MUSC (P20 RR017677). This project also was supported by the National Center for Research Resources and the Office of the Director of the NIH (C06 RR018823) to MUSC.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SEH, HL, AA, YC, JGG, LA, JD and NM provided substantial contributions to the conception and design of this study and to the acquisition of data or analysis and interpretation of data. All authors were involved in drafting the article or revising it critically for important intellectual content and gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sophie E. Hussey and Helen Lum contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 266 kb)
Rights and permissions
About this article
Cite this article
Hussey, S.E., Lum, H., Alvarez, A. et al. A sustained increase in plasma NEFA upregulates the Toll-like receptor network in human muscle. Diabetologia 57, 582–591 (2014). https://doi.org/10.1007/s00125-013-3111-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3111-x