Abstract
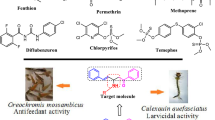
A series of chalcones (A–CH=CH–CO–B) were synthesized under microwave irradiation, and for the first time their pesticidal activity against diamondback moth (Plutella xylostella) was evaluated to identify the promising lead structures. The structure–activity relationship (SAR) analysis revealed that electron-withdrawing substituents on ring A of chalcone provided good pesticidal agents, whereas, ring B can bear either electron-withdrawing or electron-releasing substituents. Moreover, compound 22 having para-Cl substitution on ring A as well on ring B showed maximum activity with LC50 value of 170.24 μg mL−1.



Similar content being viewed by others
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. Annu Rev Entomol 18:265–267
Awasthi SK, Mishra N, Dixit SK, Singh A, Yadav M, Yadav SS, Rathaur S (2009) Antifilarial activity of 1,3-diarylpropen-1-one: effect on glutathione-S-transferase, a phase II detoxification enzyme. Am J Trop Med Hyg 80:764–768
Batovska D, Parushev S, Stamboliyska B, Tsvetkova I, Ninova M, Najdenski H (2009) Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur J Med Chem 44:2211–2218
Begum NA, Roy N, Laskar RA, Roy K (2011) Mosquito larvicidal studies of some chalcone analogues and their derived products: structure–activity relationship analysis. Med Chem Res 20:184–191
Bhardwaj A, Tewary DK, Kumar R, Kumar V, Sinha AK, Shanker A (2010) Larvicidal and structure-activity studies of natural phenylpropanoids and their semisynthetic derivatives against the tobacco armyworm, Spodoptera litura (Fab.). (Lepidoptera: Noctuidae). Chem Biodivers 7:168–177
Das BP, Begum NA, Choudhury DN, Banerji J (2005) Larvicidal studies of chalcones and their derivatives. J Indian Chem Soc 82:161–164
de la Hoz A, Díaz-Ortiz A, Moreno A (2005) Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev 34:164–178
Dimmock JR, Elias DW, Beazely MA, Kandepu NM (1999) Bioactivities of chalcones. Curr Med Chem 6:1125–1149
Dong F, Jian C, Zhenghao F, Kai G, Zuliang L (2008) Synthesis of chalcones via Claisen–Schmidt condensation reaction catalyzed by acyclic acidic ionic liquids. Catal Commun 9:1924–1927
Eskenazi B, Bradman A, Castorina R (1999) Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 107:409–413
Finney DJ (1971) Probit analysis, 3rd edn. Univ Press, Cambridge, UK
Gautam N, Chourasia OP (2010) Synthesis, antimicrobial and insecticidal activity of some new cinnoline based chalcones and cinnoline based pyrazoline derivatives. Indian J chem 49B:830–835
Geyer JA, Keenan SM, Woodard CL, Thompson PA, Gerena L, Nichols DA, Gutteridge CE, Waters NC (2009) Selective inhibition of Pfmrk, a Plasmodium falciparum CDK, by antimalarial 1,3-diaryl-2-propenones. Bioorg Med Chem Lett 19:1982–1985
Go ML, Wu X, Liu XL (2005) Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 12:483–499
Gonza′lez JA, Braun AE (1998) Effect of (E)-chalcone on potato-cyst nematodes (Globodera pallida and G. rostochiensis). J Agric Food Chem 46:1163–1165
Jin F, Jin XY, Jin YL, Sohn DW, Kim SA, Sohn DH, Kim YC, Kim HS (2007) Structural requirements of 2′,4′,6′-tris(methoxymethoxy) chalcone derivatives for anti-inflammatory activity: the importance of a 2′-hydroxy moiety. Arch Pharm Res 30:1359–1367
Kumar R, Sharma A, Sharma N, Sinha AK (2008) Neutral ionic liquid [hmim]Br as a green reagent and solvent for mild and efficient dehydration of benzyl alcohols into (E)-arylalkenes under microwave irradiation. Eur J Org Chem 5577–5582
Kumar R, Mohanakrishnan D, Sharma A, Kaushik NK, Kalia K, Sinha AK, Sahal D (2010) Reinvestigation of structure-activity relationship of methoxylated chalcones as antimalarials: synthesis and evaluation of 2,4,5-trimethoxy substituted patterns as lead candidates derived from abundantly available natural β-asarone. Eur J Med Chem 45:5292–5301
Liu M, Wilairat P, Go ML (2001) Antimalarial alkoxylated and hydroxylated chalcones: structure-activity relationship analysis. J Med Chem 44:4443–4452
Miyamoto T, Yamamoto I (1994) Glutathione conjugates as the activated form of chalcones for glutathione-S-transferase inhibition. J Pestic Sci 19:53–58
Miyata T, Saito T, Noppun V (1986) Studies on the mechanism of diamondback moth resistance of insecticides. In: Talekar NS (ed) Diamondback moth management: proc first int workshop, AVRDC, pp 347–357
Modzelewska A, Pettit C, Achanta G, Davidson NE, Huangb P, Khan SR (2006) Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg Med Chem 14:3491–3495
Nalwar YS, Sayyed MA, Mokle SS, Zanwar PR, Vibhute YB (2009) Synthesis and insect antifeedant activity of some new chalcones against Phenacoccus solanopsis. World J Chem 4:123–126
Nowakowska Z (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125–137
Rushmore TH, Pickett CB (1993) Glutathione-S-transferases, structure regulation and therapeutic implications. J Biol Chem 268:11475–11478
Santos L, Pedrosa RC, Correa R, Filho VC, Nunes RJ, Yunes RA (2006) Biological evaluation of chalcones and analogues as hypolipidemic agents. Arch Pharm Chem Life Sci 339:541–546
Schuler TH, Torres DM, Thompson AJ, Denholm I, Devonshire AL, Duce IR, Williamson MS (1998) Toxicological, electrophysiological and molecular characterization of knockdown resistance to pyrethroid insecticides in the diamondback moth, Plutella xylostella (L.). Pestic Biochem Physiol 59:169–182
Sharma A, Kumar V, Sinha AK (2006) A chemoselective hydrogenation of the olefinic bond of α, β-unsaturated carbonyl compounds in aqueous medium under microwave irradiation. Adv Synth Catal 348:354–360
Sharma A, Sharma N, Kumar R, Sharma UK, Sinha AK (2009) Water promoted cascade rearrangement approach towards α-aryl aldehydes from arylalkenes using N-halosuccinimides: an avenue for asymmetric oxidation using phase transfer cinchona organocatalysis. Chem Commun 5:299–5301
Shelton AM, Wyman JA, Cushing NL, Apfelbeck K, Dennehy TJ, Mahr SER, Eigenbrode SD (1993) Insecticide resistance of diamondback moth (Lepidoptera: Plutellidae) in North America. J Econ Entomol 86:11–19
Sinha AK, Joshi BP, Acharya R (2003) Microwave-promoted synthesis of methoxylated benzaldehydes from natural cis-phenylpropenes using NaIO4/OsO4 (cat.). Chem Lett 32:780–781
Sinha AK, Joshi BP, Dogra R (2007) One step conversion of toxic β-asarone from Acorus calamus into 1-(2,4,5-trimethoxyphenyl)-1,2-dihydroxypropane and asaronaldehyde occurring in Piper clusii. Nat Prod Res 15:439–444
Song SS (1991) Resistance of diamondback moth (Plutella xylostella L.: Yponomeutidae:Lepidoptera) against Bacillus thuringiensis Berliner. Kor J Appl Entomol 30:291–293
Sonoda S, Ashfaq M, Tsumuki H (2006) Genomic organization and developmental expression of glutathione-S-transferase genes of the diamondback moth, Plutella xylostella. J Insect Sci 6:1–9
Sun C (1990) Insecticide resistance in diamondback moth. In: Talekar NS (ed) Diamondback moth management: proc second int workshop, AVRDC, pp 419–426
Tabashnik BE, Cushing NL, Finson N, Johnson MW (1990) Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83:1671–1676
Talekar NS, Griggs TD (1986) Diamondback moth management. in Proc First International Workshop, Tainan, Taiwan
Talekar NS, Shelton AM (1993) Biology, ecology and management of the diamondback moth. Annu Rev Entomol 38:275–301
Tewary DK, Bhardwaj A, Sharma A, Sinha AK, Shanker A (2006) Bioactivity and structure–activity relationship of natural methoxylated phenylpropenes and their derivatives against Aphis craccivora Koch (Hemiptera: Aphididae). J Pest Sci 79:209–214
Verkerk RHJ, Wright DJ (1996) Multitrophic interactions and management of the diamondback moth: a review. Bull Entomol Res 86:205–216
Villarini M, Moretti M, Pasquini R, Scassellati-Sforzolini G, Fatigoni C, Marcarelli M, Monarca S, Rodríguez AV (1998) In vitro genotoxic effects of the insecticide deltamethrin in human peripheral blood leukocytes: DNA damage (‘comet’ assay) in relation to the induction of sister-chromatid exchanges and micronuclei. Toxicology 15:129–139
Zhao JZ, Collins HL, Li YX, Mau RF, Thompson GD, Hertlein MS, Andaloro JT, Boyken R, Shelton AM (2006) Monitoring of diamondback moth resistance to spinosad, indoxacarb and emamectin benzoate. J Econ Entomol 99:176–181
Acknowledgment
R. K is indebted to CSIR, New Delhi, for the award of research fellowship. The authors are thankful to Mr. S. Kumar for the technical support provide during NMR and mass spectroscopy. The authors gratefully acknowledge the financial assistance from project MLP-0025 and thank Director IHBT (CSIR), Palampur for providing the necessary infrastructure for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, P., Shard, A. et al. Chalcones as promising pesticidal agents against diamondback moth (Plutella xylostella): microwave-assisted synthesis and structure–activity relationship. Med Chem Res 21, 922–931 (2012). https://doi.org/10.1007/s00044-011-9602-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9602-8