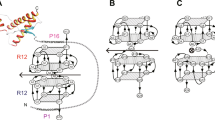
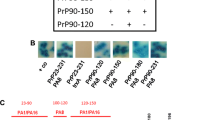
Abstract
Prion diseases are fatal neurodegenerative and infectious disorders of humans and animals, characterized by structural transition of the host-encoded cellular prion protein (PrPc) into the aberrantly folded pathologic isoform PrPSc. RNA, DNA or peptide aptamers are classes of molecules which can be selected from complex combinatorial libraries for high affinity and specific binding to prion proteins and which might therefore be useful in diagnosis and therapy of prion diseases. Nucleic acid aptamers, which can be chemically synthesized, stabilized and immobilized, appear more suitable for diagnostic purposes, allowing use of PrPSc as selection target. Peptide aptamers facilitate appropriate intracellular expression, targeting and re-routing without losing their binding properties to PrP, a requirement for potential therapeutic gene transfer experiments in vivo. Elucidation of structural properties of peptide aptamers might be used as basis for rational drug design, providing another attractive application of peptide aptamers in the search for effective anti-prion strategies.




Similar content being viewed by others
References
Dearmond SJ, Prusiner SB (1995) Etiology and pathogenesis of prion diseases. Am J Pathol 146:785–811
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Weissmann C (2004) The state of the prion. Nat Rev Microbiol 2:861–871
Aguzzi A, Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116:313–327
Collinge J (2005) Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry 76:906–919
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Cohen FE, Pan KM, Huang Z, Baldwin M, Fletterick RJ, Prusiner SB (1994) Structural clues to prion replication. Science 264:530–531
Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24:519–550
Aguzzi A, Haass C (2003) Games played by rogue proteins in prion disorders and Alzheimer’s disease. Science 302:814–818
Dobson CM (2006) Protein aggregation and its consequences for human disease. Protein Pept Lett 13:219–227
Gajdusek DC (1977) Unconventional viruses and the origin and disappearance of kuru. Science 197:943–960
Collinge J, Sidle KC, Meads J, Ironside J, Hill AF (1996) Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383:685–690
Anderson RM, Donnelly CA, Ferguson NM, Woolhouse MEJ, Watt CJ, Udy HJ, Mawhinney S, Dunstan SP, Southwood TRE, Wilesmith JW, Ryan JBM, Hoinville LJ, Hillerton JE, Austin AR, Wells GAH (1997) Transmission dynamics and epidemiology of BSE in British cattle (vol 382, pp 779, 1996). Nature 386:302–302
Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ (1997) Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501
Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P (1997) The same prion strain causes vCJD and BSE. Nature 389:448–450 526
Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J (2001) Tissue distribution of protease resistant prion protein in variant Creutzfeldt–Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180
Sigurdson CJ, Miller MW (2003) Other animal prion diseases. Br Med Bull 66:199–212
Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC (2006) Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117
Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW (2004) Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527–529
Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG (2004) Possible transmission of variant Creutzfeldt–Jakob disease by blood transfusion. Lancet 363:417–421
Caughey B, Raymond GJ (1991) The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem 266:18217–18223
Borchelt DR, Taraboulos A, Prusiner SB (1992) Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem 267:16188–16199
Westergard L, Christensen HM, Harris DA (2007) The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta 1772:629–644
Caughey B, Baron GS (2006) Prions and their partners in crime. Nature 443:803–810
McLennan NF, Brennan PM, McNeill A, Davies I, Fotheringham A, Rennison KA, Ritchie D, Brannan F, Head MW, Ironside JW, Williams A, Bell JE (2004) Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol 165:227–235
Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, Kubosaki A, Matsumoto Y, Saeki K, Matsumoto Y, Yokoyama T, Itohara S, Onodera T (1999) Prions prevent neuronal cell-line death. Nature 400:225–226
Chiarini LB, Freitas AR, Zanata SM, Brentani RR, Martins VR, Linden R (2002) Cellular prion protein transduces neuroprotective signals. EMBO J 21:3317–3326
Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas CI, Weiss S (2001) Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J 20:5876–5886
Leucht C, Simoneau S, Rey C, Vana K, Rieger R, Lasmezas CI, Weiss S (2003) The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep 4:290–295
Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G (2004) Cells release prions in association with exosomes. Proc Natl Acad Sci USA 101:9683–9688
Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol 211:582–590
Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner SB (1995) Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol 129:121–132
Vey M, Pilkuhn S, Wille H, Nixon R, Dearmond SJ, Smart EJ, Anderson RG, Taraboulos A, Prusiner SB (1996) Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc Natl Acad Sci USA 93:14945–14949
Come JH, Fraser PE, Lansbury PT Jr (1993) A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci USA 90:5959–5963
Lansbury PT (1994) Mechanism of scrapie replication. Science 265:1510
Daude N, Marella M, Chabry J (2003) Specific inhibition of pathological prion protein accumulation by small interfering RNAs. J Cell Sci 116:2775–2779
Gilch S, Winklhofer KF, Groschup MH, Nunziante M, Lucassen R, Spielhaupter C, Muranyi W, Riesner D, Tatzelt J, Schatzl HM (2001) Intracellular re-routing of prion protein prevents propagation of PrP(Sc) and delays onset of prion disease. EMBO J 20:3957–3966
Tatzelt J, Prusiner SB, Welch WJ (1996) Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J 15:6363–6373
Caspi S, Halimi M, Yanai A, Sasson SB, Taraboulos A, Gabizon R (1998) The anti-prion activity of Congo red. Putative mechanism. J Biol Chem 273:3484–3489
Chabry J, Priola SA, Wehrly K, Nishio J, Hope J, Chesebro B (1999) Species-independent inhibition of abnormal prion protein (PrP) formation by a peptide containing a conserved PrP sequence. J Virol 73:6245–6250
Horiuchi M, Baron GS, Xiong LW, Caughey B (2001) Inhibition of interactions and interconversions of prion protein isoforms by peptide fragments from the C-terminal folded domain. J Biol Chem 276:15489–15497
Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, Dwek RA, Burton DR, Prusiner SB (2001) Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739–743
Caughey B, Raymond GJ (1993) Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol 67:643–650
Zuber C, Knackmuss S, Rey C, Reusch U, Rottgen P, Frohlich T, Arnold GJ, Pace C, Mitteregger G, Kretzschmar HA, Little M, Weiss S (2008) Single chain Fv antibodies directed against the 37 kDa/67 kDa laminin receptor as therapeutic tools in prion diseases. Mol Immunol 45:144–151
Winklhofer KF, Tatzelt J (2000) Cationic lipopolyamines induce degradation of PrPSc in scrapie-infected mouse neuroblastoma cells. Biol Chem 381:463–469
Ertmer A, Gilch S, Yun SW, Flechsig E, Klebl B, Stein-Gerlach M, Klein MA, Schatzl HM (2004) The tyrosine kinase inhibitor STI571 induces cellular clearance of PrPSc in prion-infected cells. J Biol Chem 279:41918–41927
Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schatzl HM, Ertmer A (2009) Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 5:361–369
Heiseke A, Aguib Y, Riemer C, Baier M, Schatzl HM (2009) Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem 109:25–34
Priola SA, Raines A, Caughey WS (2000) Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503–1506
Forloni G, Iussich S, Awan T, Colombo L, Angeretti N, Girola L, Bertani I, Poli G, Caramelli M, Grazia BM, Farina L, Limido L, Rossi G, Giaccone G, Ironside JW, Bugiani O, Salmona M, Tagliavini F (2002) Tetracyclines affect prion infectivity. Proc Natl Acad Sci USA 99:10849–10854
Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B (1997) Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74–77
Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, Francoeur GP, Papadopoulos M, Haghighat A, Spatz SJ, Head M, Will R, Ironside J, O’Rourke K, Tonelli Q, Ledebur HC, Chakrabartty A, Cashman NR (2003) A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 9:893–899
Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, Penney M, Hegazy D, Ironside JW (2004) Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol 203:733–739
Saborio GP, Permanne B, Soto C (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810–813
Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121:195–206
Chang B, Cheng X, Yin S, Pan T, Zhang H, Wong P, Kang SC, Xiao F, Yan H, Li C, Wolfe LL, Miller MW, Wisniewski T, Greene MI, Sy MS (2007) Test for detection of disease-associated prion aggregate in the blood of infected but asymptomatic animals. Clin Vaccine Immunol 14:36–43
Brown P (2005) Blood infectivity, processing and screening tests in transmissible spongiform encephalopathy. Vox Sang 89:63–70
Dabaghian RH, Mortimer PP, Clewley JP (2004) Prospects for the development of pre-mortem laboratory diagnostic tests for Creutzfeldt-Jakob disease. Rev Med Virol 14:345–361
Gilch S, Krammer C, Schatzl HM (2008) Targeting prion proteins in neurodegenerative disease. Expert Opin Biol Ther 8:923–940
Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302:871–874
Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD, Brandner S, Jefferys JG, Collinge J (2007) Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53:325–335
Ellington AD (1994) RNA selection—aptamers achieve the desired recognition. Curr Biol 4:427–429
Gold L (1995) Oligonucleotides as research, diagnostic, and therapeutic agents. J Biol Chem 270:13581–13584
Proske D, Hofliger M, Soll RM, Beck-Sickinger AG, Famulok M (2002) A Y2 receptor mimetic aptamer directed against neuropeptide Y. J Biol Chem 277:11416–11422
Lupold SE, Hicke BJ, Lin Y, Coffey DS (2002) Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res 62:4029–4033
Li Y, Lee HJ, Corn RM (2007) Detection of protein biomarkers using RNA aptamer microarrays and enzymatically amplified surface plasmon resonance imaging. Anal Chem 79:1082–1088
Gold L, Polisky B, Uhlenbeck O, Yarus M (1995) Diversity of oligonucleotide functions. Annu Rev Biochem 64:763–797
Enari M, Flechsig E, Weissmann C (2001) Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci USA 98:9295–9299
Moroncini G, Kanu N, Solforosi L, Abalos G, Telling GC, Head M, Ironside J, Brockes JP, Burton DR, Williamson RA (2004) Motif-grafted antibodies containing the replicative interface of cellular PrP are specific for PrPSc. Proc Natl Acad Sci USA 101:10404–10409
Solforosi L, Bellon A, Schaller M, Cruite JT, Abalos GC, Williamson RA (2007) Toward molecular dissection of PrPC–PrPSc interactions. J Biol Chem 282:7465–7471
Weiss S, Proske D, Neumann M, Groschup MH, Kretzschmar HA, Famulok M, Winnacker EL (1997) RNA aptamers specifically interact with the prion protein PrP. J Virol 71:8790–8797
Proske D, Gilch S, Wopfner F, Schatzl HM, Winnacker EL, Famulok M (2002) Prion-protein-specific aptamer reduces PrPSc formation. Chembiochem 3:717–725
Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wuthrich K (1996) NMR structure of the mouse prion protein domain PrP(121–321). Nature 382:180–182
Wille H, Michelitsch MD, Guenebaut V, Supattapone S, Serban A, Cohen FE, Agard DA, Prusiner SB (2002) Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci USA 99:3563–3568
Govaerts C, Wille H, Prusiner SB, Cohen FE (2004) Evidence for assembly of prions with left-handed beta-helices into trimers. Proc Natl Acad Sci USA 101:8342–8347
Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B (2005) The most infectious prion protein particles. Nature 437:257–261
Rhie A, Kirby L, Sayer N, Wellesley R, Disterer P, Sylvester I, Gill A, Hope J, James W, Tahiri-Alaoui A (2003) Characterization of 2’-fluoro-RNA aptamers that bind preferentially to disease-associated conformations of prion protein and inhibit conversion. J Biol Chem 278:39697–39705
Sayer NM, Cubin M, Rhie A, Bullock M, Tahiri-Alaoui A, James W (2004) Structural determinants of conformationally selective, prion-binding aptamers. J Biol Chem 279:13102–13109
Sekiya S, Noda K, Nishikawa F, Yokoyama T, Kumar PK, Nishikawa S (2006) Characterization and application of a novel RNA aptamer against the mouse prion protein. J Biochem 139:383–390
Wopfner F, Weidenhofer G, Schneider R, von Brunn A, Gilch S, Schwarz TF, Werner T, Schatzl HM (1999) Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J Mol Biol 289:1163–1178
Gabus C, Derrington E, Leblanc P, Chnaiderman J, Dormont D, Swietnicki W, Morillas M, Surewicz WK, Marc D, Nandi P, Darlix JL (2001) The prion protein has RNA binding and chaperoning properties characteristic of nucleocapsid protein NCP7 of HIV-1. J Biol Chem 276:19301–19309
Gomes MP, Millen TA, Ferreira PS, Cunha E, Silva NL, Vieira TC, Almeida MS, Silva JL, Cordeiro Y (2008) Prion protein complexed to N2a cellular RNAs through its N-terminal domain forms aggregates and is toxic to murine neuroblastoma cells. J Biol Chem 238:19616–19625
Mercey R, Lantier I, Maurel MC, Grosclaude J, Lantier F, Marc D (2006) Fast, reversible interaction of prion protein with RNA aptamers containing specific sequence patterns. Arch Virol 151:2197–2214
Takemura K, Wang P, Vorberg I, Surewicz W, Priola SA, Kanthasamy A, Pottathil R, Chen SG, Sreevatsan S (2006) DNA aptamers that bind to PrP(C) and not PrP(Sc) show sequence and structure specificity. Exp Biol Med (Maywood) 231:204–214
Kouassi GK, Wang P, Sreevatan S, Irudayaraj J (2007) Aptamer-mediated magnetic and gold-coated magnetic nanoparticles as detection assay for prion protein assessment. Biotechnol Prog 23:1239–1244
Bibby DF, Gill AC, Kirby L, Farquhar CF, Bruce ME, Garson JA (2008) Application of a novel in vitro selection technique to isolate and characterise high affinity DNA aptamers binding mammalian prion proteins. J Virol Methods 151:107–115
Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB (1998) Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–1165
Pfeifer A, Eigenbrod S, Al Khadra S, Hofmann A, Mitteregger G, Moser M, Bertsch U, Kretzschmar H (2006) Lentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected mice. J Clin Invest 116:3204–3210
White MD, Farmer M, Mirabile I, Brandner S, Collinge J, Mallucci GR (2008) Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc Natl Acad Sci USA 105:10238–10243
Blind M, Kolanus W, Famulok M (1999) Cytoplasmic RNA modulators of an inside-out signal-transduction cascade. Proc Natl Acad Sci USA 96:3606–3610
Colas P, Cohen B, Jessen T, Grishina I, Mccoy J, Brent R (1996) Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature 380:548–550
Hoppe-Seyler F, Crnkovic-Mertens I, Tomai E, Butz K (2004) Peptide aptamers: Specific inhibitors of protein function. Curr Mol Med 4:529–538
Ladner RC (1995) Constrained peptides as binding entities. Trends Biotechnol 13:426–430
Geyer CR, Colman-Lerner A, Brent R (1999) “Mutagenesis” by peptide aptamers identifies genetic network members and pathway connections. Proc Natl Acad Sci USA 96:8567–8572
Baines IC, Colas P (2006) Peptide aptamers as guides for small-molecule drug discovery. Drug Discov Today 11:334–341
Peelle B, Gururaja TL, Payan DG, Anderson DC (2001) Characterization and use of green fluorescent proteins from Renilla mulleri and Ptilosarcus guernyi for the human cell display of functional peptides. J Protein Chem 20:507–519
Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren PA (1997) Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol 15:772–777
Beste G, Schmidt FS, Stibora T, Skerra A (1999) Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc Natl Acad Sci USA 96:1898–1903
Colas P, Cohen B, Ferrigno PK, Silver PA, Brent R (2000) Targeted modification and transportation of cellular proteins. Proc Natl Acad Sci USA 97:13720–13725
Butz K, Denk C, Fitscher B, Crnkovic-Mertens I, Ullmann A, Schroder CH, Hoppe-Seyler F (2001) Peptide aptamers targeting the hepatitis B virus core protein: a new class of molecules with antiviral activity. Oncogene 20:6579–6586
Butz K, Denk C, Ullmann A, Scheffner M, Hoppe-Seyler F (2000) Induction of apoptosis in human papillomavirus-positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc Natl Acad Sci USA 97:6693–6697
Nauenburg S, Zwerschke W, Jansen-Durr P (2001) Induction of apoptosis in cervical carcinoma cells by peptide aptamers that bind to the HPV-16 E7 oncoprotein. FASEB J 15:592–594
Buerger C, Nagel-Wolfrum K, Kunz C, Wittig I, Butz K, Hoppe-Seyler F, Groner B (2003) Sequence-specific peptide aptamers, interacting with the intracellular domain of the epidermal growth factor receptor, interfere with Stat3 activation and inhibit the growth of tumor cells. J Biol Chem 278:37610–37621
Gilch S, Kehler C, Schatzl HM (2007) Peptide aptamers expressed in the secretory pathway interfere with cellular PrPSc formation. J Mol Biol 371:362–373
Wuertzer CA, Sullivan MA, Qiu X, Federoff HJ (2008) CNS delivery of vectored prion-specific single-chain antibodies delays disease onset. Mol Ther 16:481–486
Siernion IZ, Cebrat M, Kluczyk A (2004) The problem of amino acid complementarity and antisense peptides. Curr Protein Pept Sci 5:507–527
Root-Bernstein RS, Holsworth DD (1998) Antisense peptides: a critical mini-review. J Theor Biol 190:107–119
Huang YY, Zhao R, Luo J, Xiong SX, Shangguan DH, Zhang HW, Liu GQ, Chen Y (2008) Design, synthesis and screening of antisense peptide based combinatorial peptide libraries towards an aromatic region of SARS-CoV. J Mol Recognit 21:122–131
Imai M, Baranyi L, Okada N, Okada H (2007) Inhibition of HIV-1 infection by synthetic peptides derived CCR5 fragments. Biochem Biophys Res Commun 353:851–856
Rother KI, Clay OK, Bourquin JP, Silke J, Schaffner W (1997) Long non-stop reading frames on the antisense strand of heat shock protein 70 genes and prion protein (PrP) genes are conserved between species. Biol Chem 378:1521–1530
Moser M, Oesch B, Bueler H (1993) An anti-prion protein? Nature 362:213–214
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilch, S., Schätzl, H.M. Aptamers against prion proteins and prions. Cell. Mol. Life Sci. 66, 2445–2455 (2009). https://doi.org/10.1007/s00018-009-0031-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-009-0031-5