Abstract
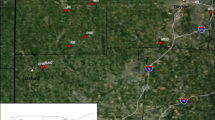
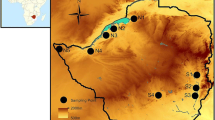
Hemerocallis hakuunensis, a Korean endemic species, maintains considerably higher levels of allozyme variation within populations (meanHe=0.253) and substantially lower levels of allozyme divergence among populations (meanG ST=0.077) than average values reported for other insect-pollinated, outcrossing herbs. Indirect estimates of the number of migrants per generation (Nm=3.00, calculated fromG ST;Nm=3.57, calculated from the frequency of nine alleles unique to single populations) indicate that gene flow has been extensive inH. hakuunensis. This is somewhat surprising when we consider the fact that no specialized seed dispersal mechanism is known, flowers are visited by bees, and the present-day populations of the species are discontinous and isolated. Results of a spatial autocorrelation analysis based on mean allele frequencies of 19 populations reveal that only 13% (95/720 cases) of Moran'sI values for the ten interpopulational distance classes are significantly different from the expected values and no distinct trend with respect to the distance classes is detected. Although it is unclear how the populations are genetically homogenous, it is highly probable thatH. hakuunensis might have a history of relatively large, continuous populations that had more chance for gene movement among adjacent populations after the last Ice Age. In addition, occasional hybridization withH. thunbergii in areas of sympatry in the central and southwestern Korean Peninsula may be one factor contributing the present-day high allozyme variation observed inH. hakuunensis.
Similar content being viewed by others
References
Barton, N.H. andSlatkin, M. 1986. A quasi-equilibrium theory of the distribution of rare alleles in a subdivided population. Heredity56: 409–415.
Broyles, S.B. andWyatt, R. 1993. Allozyme diversity and genetic structure in southern Appalachian populations of poke milkweed,Asclepias exaltata. Syst. Bot.18: 18–30.
Cheliak, W.M. andPitel, J.A. 1994. Techniques for starch gel electrophoresis of enzymes from forest species.Information Report PI-42, Petawawa National Forestry Institute, Agriculture Canada, Canadian Forestry Service, Pp. 1–49. Chalk River, Ontario.
Chung, M.G. 1994. Genetic variation and population structure in Korean endemic species: III.Hosta minor (Liliaceae). J. Plant Res.107: 377–383.
Chung, M.G. 1995. Genetic diversity in two island endemics:Hosta venusta andH. tsushimensis (Liliaceae). J. Jap. Bot.70: 322–327.
Chung, M.G. 1996. Spatial genetic structure among Korean populations ofHosta minor andH. capitata (Liliaceae). Bot. Bull. Acad. Sin.37: 25–30.
Chung, M.G., Hamrick, J.L., Jones, S.B., andDerda, G.S. 1991. Isozyme variation within and among populations ofHosta (Liliaceae) in Korea. Syst. Bot.16: 667–684.
Chung, M.G. andKang S.S. 1994a. Morphometric analysis of the genusHemerocallis L (Liliaceae) in Korea. J. Plant Res.107: 165–175.
Chung, M.G. andKang, S.S. 1994b. Genetic variation and population structure in populations ofEurya japonica (Theaceae) in Korea. Amer. J. Bot.81: 1077–1082.
Chung, M.G. andKang, S.S. 1995. Spatial genetic structure among Korean populations ofEurya japonica andE. emarginata (Theaceae). Ann. Bot. Fennici.32: 233–237.
Clayton, J.W. andTretiak, D.N. 1972. Amine-citrate buffers for pH control in starch gel electrophoresis. J. Fish. Res. Board Can.29: 1169–1172.
Cliff, A.D. andOrd, J.K. 1981. Spatial Process-Models and Applications. Pion, London.
Cole, C.T. andBiesboer, D.D. 1992. Monomorphism, reduced gene flow, and cleistogamy in rare and common species ofLespedeza (Fabaceae). Amer. J. Bot.79: 567–575.
Cosner, M.E. andCrawford, D.J. 1994. Comparisons of isozyme diversity in three rare species ofCoreopsis (Asteraceae). Syst. Bot.19: 350–358.
Dewey, S.E. andHeywood, J.S. 1988. Spatial autocorrelation in a population ofPsychotria nervosa. I. Distribution of genotypes. Evolution47: 834–838.
Epperson, B.K. andClegg, M.T. 1986. Spatial autocorrelation of flower color polymorphisms within substructured populations of morning glory (Ipomea purpurea). Am. Nat.128: 840–858.
Fowler, D.P. andMorris, R.W. 1977. Genetic diversity in red pine: evidence for low genetic heterozygosity. Can. J. For. Res.7: 343–347.
Godt, M.J.W. andHamrick, J.L. 1993. Genetic diversity and population structure inTradescantia hirsuticaulis (Commelinaceae). Amer. J. Bot.80: 959–966.
Godt, M.J.W. andHamrick, J.L. 1996. Genetic structure of two endangered pitcher plants,Sarracenia jonesii andS. oreophila (Sarraceniaceae). Amer. J. Bot.83: 1016–1023.
Godt, M.J.W., Hamrick, J.L. andBratton, S. 1995. Genetic diversity in a threatened wetland species,Helonias bullata (Liliaceae). Conserv. Biol.9: 596–604.
Hamrick, J.L. andGodt, M.J.W. 1989. Allozyme diversity in plant species.In A.D.H. Brown, M.T. Clegg, A.L. Kahler, and B.S. Weir, eds., Plant Population Genetics, Breeding and Genetic Resources. Sinauer, Sunderland, pp. 43–63.
Hamrick, J.L., Godt, M.J.W., Murawski, D.A. andLoveless, M.D. 1991. Correlations between species traits and allozyme diversity: Implications for conservation biology.In D.A. Falk and K.E. Holsinger, eds., Genetics and Conservation of Rare Plants. Oxford Univ. Press, New York, pp. 76–86.
Hamrick, J.L., Godt, M.J.W. andSherman-Broyles, S.L. 1992. Factors influencing levels of genetic diversity in woody plant species. New Forests6: 95–124.
Hartl, D.L. andClark, A.G. 1989. Principles of Population Genetics. Sinauer, Sunderland, MA.
Haufler, C.H. 1985. Enzyme variability and modes of evolution inBommeria (Pteridaceae). Syst. Bot.10: 92–104.
Hiebert, R.D. andHamrick, J.L. 1983. Patterns and levels of genetic variation in Great Basin Bristlecone pine,Pinus longaeva. Evolution37: 302–310.
Heywood, J.S. 1991. Spatial analysis of genetic variation in plant populations. Annu. Rev. Ecol. Syst.22: 335–355.
Holsinger, K.E. andGottlieb, L.D. 1991. Conservation of rare and endangered plants: Principles and prospects.In D.A. Falk and K.E. Holsinger, eds., Genetics and Conservation of Rare Plants. Oxford Univ. Press, New York, pp. 195–208.
Hotta, M., Ito, M. andOkada, I. 1984. Anthesis of the genusHemerocallis and its variation. Special mentions to nocturnalH. thunbergii of Tsushima & Hirado Islands, Western Japan. Acta Phytotax. Geobot.35: 84–93.
Hotta, M., Ito, M. andOkada, I. 1985. Differentiation and species relationships of island populations ofHemerocallis around Kyushu, Japan.In H. Hara, ed. Origin and Evolution of Diversity in Plants and Plant Communities. Acad. Sci. Book Inc., Tokyo. pp. 18–31.
Jensen, R.T. 1986. Geographic spatial autocorrelation inQuercus ellipsoidalis. Bull. Torrey Bot. Club.113: 431–439.
Kang, S.S. andChung M.G. 1994.Hemerocallis hakuunensis (Liliaceae) in Korea. Sida16: 23–31.
Karron, J.D., Linhart, Y.B., Chaulk, C.A. andRobertson, C.A. 1988. The genetic structure of populations of geographically restricted and widespread species ofAstragalus (Fabaceae). Amer. J. Bot.75: 1114–1119.
Karron, J.D. 1991. Patterns of genetic variation and breeding systems in rare plant species.In D.A. Falk and K.E. Holsinger, eds., Genetics and Conservation of Rare Plants. Oxford. Univ. Press, New York, pp. 87–98.
Kawano, S. 1961. On the natural hybrid population ofHemerocallis. Can. J. Bot.39: 667–681.
Lewis, P.O. 1991. Allozyme variation in the rare Gulf Coast endemicPolygonella macrophylla (Polygonaceae). Plant Species Biol.6: 1–10.
Lewis, P.O. andCrawford, D.J. 1995. Pleistocene refugium endemics exhibit greater allozymic diversity than widespread congeners in the genusPolygonella (Polygonaceae). Amer. J. Bot.82: 141–149.
Li, C.C. andHorvitz, D.G. 1953. Some methods of estimating the inbreeding coefficient. Amer. J. Human Genet.5: 107–117.
Linhart, Y.B. andPremoli, A.C. 1993. Genetic variation inAletes acaulis and its relative, the narrow endemicA. humilis (Apiaceae). Amer. J. Bot.80: 598–605.
Mitton, J.B., Linhart, Y.B., Sturgeon, K.B. andHamrick, J.L. 1979. Allozyme polymorphisms detected in mature needle tissue of ponderosa pine. J. Hered.70: 86–89.
Nakao, S. and Yamashita, K. 1956. Variation in some plant populations.In T. Komai and K. Sakai, eds., Syundan Idengaku (Population Genetics) Tokyo (in Japanese), pp. 248–254.
Nei, M. 1972. Genetic distance between populations. Am. Nat.106: 283–292.
Nei, M. 1973. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA70: 3321–3323.
Nei, M. 1977.F-statistics and analysis of gene diversity in subdivided populations. Ann. Human Genet.41: 225–233.
Noguchi, J. 1986. Geographical and ecological differentiation in theHemerocallis dumortieri complex with species reference to its karyology. J. Sci. Hiroshima Univ. Ser B. Div. 2, Bot.20: 29–193.
Olmstead, R.G. 1990. Biological and historical factors influencing genetic diversity in theScutellaria angustifolia (Liliaceae). Evolution44: 54–70.
Purdy, B.G. andBayer, R.J. 1995. Genetic diversity in the tetraploid sand dune endemicDeschampsia mackenzieana and its widespread diploid progenitorD. cespitose (Poaceae). Amer. J. Bot.82: 121–130.
Purdy, B.G., Bayer, R.J. andMacDonald. 1994. Genetic variation, breeding system, evolution and conservation of the narrow sand dune endemicStellaria arenicola and the widespreadS. longipes (Caryophyllaceae). Amer. J. Bot.81: 904–911.
Rohlf, F.J. 1988. Numerical Taxonomy and Multivariate Analysis System. Exeter Publishing, Setauket, NY.
Sakai, A.K. andOden, N.L. 1983. Spatial pattern of sex expression in silver maple (Acer saccharium L.): Morista's index and spatial autocorrelation. Am. Nat.122: 489–508.
Sherman-Broyles, S.L., Gibson, J.P., Hamrick, J.L., Bucher, M.A. andGibson, M.J. 1992. Comparisons of allozyme diversity among rare and widespreadRhus species. Syst. Bot.17: 551–559.
Slatkin, M. 1985. Rare alleles as indicators of gene flow. Evolution39: 53–65.
Sokal, R.R., Crovello, T.J. andUnnasch, R. 1986. Geographic variation of vegetative characters ofPopulus dettoides. Syst. Bot.11: 419–432.
Sokal, R.R. andOden, N.L. 1978a. Spatial autocorrelation in biology. 1. Methodology. Biol. J. Linn. Soc.10: 199–249.
Sokal, R.R. andOden, N.L. 1978b. Spatial autocorrelation in biology. 2. Some biological implications and four applications of evolutionary and ecological interest. Biol. J. Linn. Soc.10: 229–249.
Soltis, D.E. 1982. Allozyme variability inSullivantia (Saxifragacea). Syst. Bot.7: 26–34.
Soltis, D.E., Haufler, C.H., Darrow, D.C. andGastony, G.J. 1983. Starch gel electrophoresis of ferns: A compilation of grinding buffers, and staining schedules. Amer. Fern J.73: 9–27.
Soltis, P.S. andSoltis, D.E. 1991. Genetic variation in endemic and widespread plant species: Examples from Saxifragaceae andPolystichum (Dryopteridaceae). Aliso13: 215–223.
Soltis, P.S., Soltis, D.E., Tucker, T.L. andLang, F.A. 1991. Allozyme variability is absent in the narrow endemicBensoniella oregona (Saxifragaceae). Conser. Biol.6: 131–134.
Sytsma, K.J. andSchaal, B.A. 1985. Genetic variation, differentiation, and evolution in a species complex of tropical shrubs based on isozymic data. Evolution39: 582–593.
Weeden, N.F. andWendel, J.F. 1989. Genetics of plant isozymes.In D.E. Soltis and P.S. Soltis, eds. Isozymes in Plant Biology. Dioscorides Press, Portland, OR, pp. 46–72.
Workman, P.L. andNiswander, J.D. 1970. Population studies on southwestern Indian tribes. II. Local genetic differentiation in the Papago. Amer. J. Human Genet.22: 24–49.
Wright, S. 1951. The genetic structure of populations. Ann. Eugen.15: 313–354.
Wright, S. 1965. The interpretation of population structure byF-statistics with special regard to systems of mating. Evolution19: 395–420.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kang, S.S., Chung, M.G. Genetic variation and population structure in Korean endemic species: IV.Hemerocallis hakuunensis (Liliaceae). J. Plant Res. 110, 209–217 (1997). https://doi.org/10.1007/BF02509309
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02509309