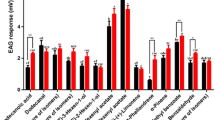
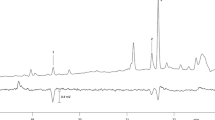
Abstract
The sex attractant ofScrobipalpuloides absoluta females is a 90:10 mixture of (3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate and (3E,8Z)-3,8-tetradecadien-1-yl acetate. Tetradecadienyl acetates bearing 8Z,11Z; 3E,8Z; and 3E,11Z double bonds were synthesized by stereospecific procedures; the mass spectral and gas chromatographic properties of the 3E,8Z isomer were found to be congruent with those of the tetradecadienyl acetate fromS. absoluta. In wind tunnel bioassays, a 10:1 mixture of synthetic (3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate and (3E,8Z)-3,8-tetradecadien-1-yl acetate was highly attractive toS. absoluta males. Interestingly, the presence of (8Z,11Z)-8,11-tetradecadien-1-yl acetate (10%) inhibited the response to (3E,8Z,11Z)-3,8,11-tetradecatrien-1-yl acetate completely.
Similar content being viewed by others
References
Arn, H., Tóth, M., andPriesner, E. 1992. List of sex pheromones of Lepidoptera and related attractants, 2nd Edition, International Organization for Biological Control, Montfavet.
Attygalle, A. B., Jham, G. N., Svatoš, A., Frighetto, R. T. S., Meinwald, J., Vilela, E. F., Ferrara, F. A., andUchoa-Fernandes, M. A. 1995. Microscale, random reduction: Application to the characterization of (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, a new lepidopteran sex pheromone.Tetrahedron Lett. 36:5471–5474.
Attygalle, A. B., Jham, G. N., Savtoš, A., Frighetto, R. T. S., Ferrara, F. A., Vilela, E. F., Uchoa-Fernandes, M. A., andMeinwald, J. 1996. (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, a sex attractant for the tomato pestScrobipalpuloides absoluta (Lepidoptera: Gelechiidae).Bioorg. Med. Chem. In press.
Bestmann, H. J., andGunawardena, N. A. 1992. Pheromones: 87. An efficient synthesis of (6E,11E)-6,11-hexdecadienyl acetate and (6E,11E)-6,11-hexadecadienal: Female sex pheromone ofAntheraea pernyi andA. polyphemus (Lepidoptera: Saturniidae).Synthesis 1992:1239–1241.
Brown, C. A., andAhuja, V. K. 1973. Catalytic hydrogenation. VI. The reaction of sodium borohydride with nickel salts in ethanol solution. P-2 nickel, a highly convenient, new, selective hydrogenation catalysts with great sensitivity to substrate structure.J. Org. Chem. 38:2226–2230.
Jung, M. E., andLyster, M. A. 1977. Quantitative dealkylation of alkyl ethers via treatment with trimethylsilyl iodide. A new method for ether hydrolysis.J. Org. Chem. 42:3761–3764.
Kang, S.-K., Kim, W.-S., andMoon, B.-H. 1985. An effective method for the preparation ofω-bromoalkanols fromα,ω-diols.Synthesis 1985:1161–1162.
Lipschutz, B. H., Wilhelm, R. S., Kozlowski, J. A., andParker, D. 1984. Substitution reactions of secondary halides and epoxides with higher order, mixed organocuprates, R2Cu(CN)Li2: Synthetic, stereochemical, and mechanistic aspects.J. Org. Chem. 49:3928–3938.
Mayer, M. S., andMcLaughlin, J. R. 1991. Handbook of Insect Pheromones and Sex Attractants, CRC Press, Boca Raton, Florida.
Miyashita, M., Yoshikoshi, A., andGrieco, P. A. 1977. Pyridiniump-toluenesulfonate: A mild and efficient catalyst for the tetrahydropyranylation of alcohols.J. Org. Chem. 42:3772–3774.
Rossi, R., andCarpita, A. 1977. Insect pheromones. Stereoselective reduction ofβ- andω-alkynols to the corresponding (E)-alkenols by lithium tetrahydroaluminate.Synthesis 1977:561–562.
Robertson, D. N. 1960. Adducts oftert-alcohols containing an ethynyl group with dihydropyran. Potentially useful intermediates.J. Org. Chem. 25:931–932.
Tamura, M., andKochi, J. 1971. Coupling of Grignard reagents with organic halides.Synthesis 1960:303–305.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Svatoš, A., Attygalle, A.B., Jham, G.N. et al. Sex pheromone of tomato pestScrobipalpuloides absoluta (Lepidoptera: Gelechiidae). J Chem Ecol 22, 787–800 (1996). https://doi.org/10.1007/BF02033586
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02033586