Summary
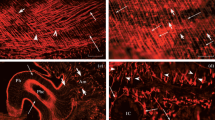
Melanophores, xanthophores, and iridophores from the skins of the two Antarctic fish speciesPagothenia borchgrevinki andTrematomus bernacchii were tested immunocytochemically for the presence of a variety of muscle proteins. Actin, myosin, and calmodulin, not surprisingly, were confirmed for all three chromatophore types of the two fishes, but the presence of caldesmon and calponin, both characteristic proteins of smooth muscle fibers, represents a new discovery. It is not known at this stage whether these proteins occur also in the chromatophores of other fishes and are not restricted to Antarctic species. Since, however, motility control of particles in fish chromatophores and the regulation of smooth muscle tension both involve the sympathetic nervous system, the presence of similar target proteins should not come as a surprise. The fact that none of the chromatophores tested positive for troponin shows that there is no close relationship between pigment cells and striated muscle. The lack of alpha-actinin in iridophores, but its presence in melanophores and xanthophores, is thought to be a reflection of the considerably greater pigment translocations within the latter two types of chromatophore cells.
Similar content being viewed by others
References
Abe M, Takahashi K, Hiwada K (1990) Effect of calponin on actin-activated myosin ATPase activity. J Biochem 108: 835–838
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1994) Molecular biology of the cell. Garland, New York
Arimura C, Suzuki T, Yanagisawa M, Imamura M, Hamada Y, Masaki T (1988) Primary structure of chicken skeletal muscle and fibroblast alpha-actinin deduced from cDNA sequences. Eur J Biochem 177: 649–655
Barany K, Polyak E, Barany M (1992) Involvement of calponin and caldesmon in sustained contraction of arterial smooth muscle. Biochem Biophys Res Commun 187: 847–852
Bartegi A, Fattoum A, Derancourt J, Kassab R (1990) Characterization of the carboxyl-terminal 10-kDa cyanogen bromide fragment of caldesmon as an actin-calmodulin binding region. J Biol Chem 265: 15231–15238
Bendayan M, Zollinger M (1983) Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A gold technique. J Histochem Cytochem 31: 101–109
Bennett JP, Zaner KS, Stossell TP (1984) Isolation and some properties of macrophages alpha-actinin: evidence that it is not an actin gelliging protein. Biochemistry 23: 5081–5086
Bikle D, Tilney LG, Porter KR (1966) Microtubules and pigment migration in the melanophores ofFundulus heteroclitus. Protoplasma 61: 322–345
Bonet-Kerrache A, Mornet D (1995) Importance of the C-terminal part of actin in interactions with calponin. Biochem Biophys Res Commun 206: 127–132
Burridge K, Feramisco J (1981) Non-muscle alpha actinins are calcium sensitive actin binding proteins. Nature 294: 565–567
Cheney RE, Riley MA, Mooseker MS (1993) Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton 24: 215–223
Collins JH (1976) Structure and evolution of troponin-C and related proteins. Soc Exp Biol Symp 30: 303–307
Dietrich HW, Himes RH (1989) Treadmilling of Antarctic fish microtubules at low temperatures. Antarctic J US 24: 196–197
Duchaiman AS, Bamburg JR (1984) Isolation of brain alpha-actinin, its characterisation and comparison of its properties with those of muscle alpha-actinins. Biochemistry 23: 1600–1608
Fujii R (1993) Cytophysiology offish chromatophores. Int Rev Cytol 143: 191–255
—, Wakatabi H, Oshima N (1991) Inositol 1,4,5-triphosphate signals the motile response of fish chromatophores I: aggregation of pigment in theTilapia melanophore. J Exp Zool 259: 9–17
Gimona M, Herzog K, Vandekerckhove J, Small JV (1990) Smooth muscle specific expression of calponin. FEBS Lett 274: 159–162
Hayashi K, Fujiio Y, Kato I, Sobue K (1991) Structural and functional relationships between h- and 1-caldesmons. J Biol Chem 266: 355–361
Hemric ME, Lu FWM, Shrager R, Carey J, Chalovich JM (1993) Reversal of caldesmon binding to myosin with calcium-calmodulin or by phosphorylating caldesmon. J Biol Chem 268: 15305–15311
Hennessey AS, Drummond DR, Sparrow JC (1993) Molecular genetics of actin function. Biochem J 282: 657–671
Ip W, Murphy DB, Heuser JE (1984) Arrest of pigment granule motion in erythrophores by quick-freezing. J Ultrastruct Res 86: 162–175
Kawai I (1989) Relation between light-sensitive response of carotenoid droplet and calcium on a scale xanthophore of the medaka,Oryzias latipes. Med Biol 118: 141–145 (in Japanese)
Korn ED, Carlier MF, Pantaloni D (1987) Actin polymerization and ATP hydolysis. Science 238: 638–644
Landon F, Gache Y, Touitou H, Olomucki A (1985) Properties of two isoforms of human blood platelet alpha-actinins. Eur J Biochem 153: 231–237
Littlepage JL (1965) Oceanography, investigations in McMurdo Sound, Antarctica. Antarct Res Ser 5: 1–37
Marston SB, Huber PA (1996) Caldesmon. In: Barany M (ed) Biochemistry of smooth muscle contraction. Academic Press, San Diego, pp 77–90
—, Redwood CS (1991) The molecular anatomy of caldesmon. Biochem J 279: 1–16
McNiven MA, Ward JB (1988) Calcium regulation of pigment transport in vitro. J Cell Biol 106: 111–125
Meyer-Rochow VB (2001) Fish chromatophores as sensors of environmental stimuli. In: Kapoor BG (ed) Sensory biology of jawed fishes: new insights. Oxford and IBH Publishing, New Delhi, M/S Science Publisher, Enfield, NH, pp 317–334
Nilsson H, Wallin M (1997) Evidence for several roles of dynein in pigment transport in melanophores. Cell Motil Cytoskeleton 38: 397–409
Noda S, Ito M, Watanabe S, Takahashi K, Maruyama K (1992) Conformational changes of actin induced by calponin. Biochem Biophys Res Commun 185: 481–487
Obika M (1986) Intracellular transport of pigment granules in fish chromatophores. Zool Sci 3: 1–11
—, Meyer-Rochow VB (1986) Ultrastructure of microtubules in dermal melanophores and spinal nerve of the Antarctic teleostPagothenia borchgrevinki. Cell Tissue Res 244: 339–343
— — (1990) Dermal and epidermal chromatophores of the Antarctic teleostTrematomus bernacchii. Pigment Cell Res 3: 33–37
Ohtsuki T, Maruyama K, Ebashi S (1986) Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem 38: 1–67
Oshima N, Suzuki M, Yamaji N, Fujii R (1988) Pigment aggregation is triggered by an increase in free calcium ions within the fish chromatophores. Comp Biochem Physiol 91A: 27–32
—, Hayakawa M, Sugimoto M (1990) The involvement of calmodulin in motile activities of fish chromatophores. Comp Biochem Physiol 97C: 33–36
—, Sekine H, Tanooka M (1998) Involvement of Ca2+ in the direct effect of K+ on xanthophores of the medakaOryzias latipes. Zool Sci 15: 645–650
Pittenger MF, Kazzaz JA, Helfman DM (1994) Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol 6: 96–104
Pollard TD, Cooper JA (1986) Actin and actin-binding proteins: a critical evaluation of mechanisms and functions. Annu Rev Biochem 55: 987–1035
Rodionov VI, Gyoeva FK, Gelfand VI (1991) Kinesin is responsible for centrifugal movement of pigment granules in melanophores. Proc Natl Acad Sci USA 88: 4956–4960
Royuela M, Astier C, Fraile B, Paniagua R (1999) Alpha-actinin in different invertebrate muscle cell types ofDrosophila melanogaster, the earthwormEisenia foetida, and the snailHelix aspersa. J Muscle Res Cell Motil 20: 1–9
—, Fraile B, Arenas MI, Paniagua R (2000) Characterization of several invertebrate muscle cell types: a comparison with vertebrate muscle. Microsc Res Tech 48: 107–115
Schliwa M (1984) Mechanisms of intracellular organelle transport. Cell Muscle Motil 5: 1–82
Shirinsky VP, Biryokov KG, Hettasch JM, Sellers JR (1992) Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem 267: 15886–15892
Sobue K, Sellers JR (1991) Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem 266: 12115–12118
Stull JT, Krueger JK, Kamm KE, Gao Z, Zhi G, Padre R (1996) Myosin light chain kinase. In: Barany M (ed) Biochemistry of smooth muscle contraction. Academic Press, San Diego, pp 119–130
Szymanski PT, Tao T (1993) Interaction between calponin and smooth muscle myosin. FEBS Lett 334: 379–382
Takahashi K, Nadal-Ginard B (1991) Molecular cloning and sequence analysis of smooth muscle calponin. J Biol Chem 266: 13284–13288
Titus MA (1993) Myosins. Curr Opin Cell Biol 5: 77–81
Winder SJ, Walsh MP (1990) Smooth muscle calponin: inhibition of actomyosin Mg-Atpase and regulation by phosphorylation. J Biol Chem 265: 10148–10155
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer-Rochow, V.B., Royuela, M., Fraile, B. et al. Smooth muscle proteins as intracellular components of the chromatophores of the Antarctic fishesPagothenia borchgrevinki andTrematomus bernacchii (Nototheniidae). Protoplasma 218, 24–30 (2001). https://doi.org/10.1007/BF01288357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01288357