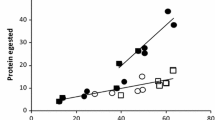
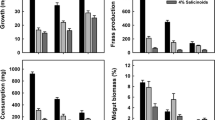
Summary
The midgut pH of late instar gypsy moth (Lymantria dispar L.) larvae is strongly alkaline, and varies with diet, larval stadium, and time since feeding. Midgut pH rises with time since feeding, and does so more quickly, reaching greater maximum values, on some diets than others. Leaf tissues of 23 tree species resist increases in alkalinity differentially; this trait and differing initial leaf pH may explain the impact of diet on gut pH. Third instar larvae may have gut conditions favorable for tannin-protein binding shortly after ingesting certain foods, but with time midgut alkalinity becomes great enough to dissociate tannin-protein complexes. Older instars rarely exhibit gut pHs low enough to permit tannin activity. Alkaline gut conditions may explain the gypsy moth's ability to feed on many tanniniferous plant species, especially in later instars. Consequences for pathogen effectiveness are discussed.
Similar content being viewed by others
References
Berenbaum M (1980) Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115:138–146
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Brooks GT (1977) Epoxide hydratase as a modifier of biotransformation and biological activity. Gen Pharmacol 8:221–226
Chapman RF (1971) The Insects — Structure and Function. American Elsevier, NY, USA
Crawley MJ (1983) The Dynamics of Animal-Plant Interactions, University of California Press, Berkeley, CA, USA
Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10:1–40
Lance DR, Elkinton JS, Mastro VC, Schwalbe CP (1983) APHIS OTIS Methods Dey Ctr Annual Report, USDA, Otis Air National Guard Base, MA, USA
Lechowicz MJ (1983) Leaf quality and the host preference of gypsy moth in the northern decidous forest, in: Talerico RL, Montgomery M (eds) Forest Defoliator-Host Interactions: A Comparison Between Gypsy Moth and Spruce Budworms. USDA Forest Service General Technical Report NE-85. Northeastern Forest Experiment Station, Broomall, PA, USA, pp 67–82
Leonard DE (1970) Feeding rhythm in larvae of the gypsy moth. J Econ Entomol 63:1454–1457
Loomis WD, Bataille J (1966) Plant phenolic compounds and the isolation of enzymes. Phytochemistry 5:423–438
Lowry P, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martin JS, Martin MM (1983) Precipitation of ribuluse-1, S-bisphosphate carboxylase/oxygenase by tannic acid, quebracho, and oak foliage extracts. J Chem Ecol 9:285–294
Martin MM, Martin JS (1984) Surfactants: their role in preventing the precipitation of proteins by tannins in insect guts. Oecologia (Berlin) 61:342–345
Maufette Y, Lechowicz MJ, Jobin L (1983) Host preferences of the gypsy moth, Lymantria dispar (L.), in Sothern Quebec. Can J For Res 13:53–60
Raven JA, Smith FA (1976) Cytoplasmic pH regulation and electrogenig H+ extrusion. Curr Adv Plant Sci 8:649–660
Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH (eds) Herbivores: Their Interaction With Secondary Plant Metabolites, Academic Press, NY, USA, pp 5–54
Schultz JC (1983) Impact of variable plant chemical defenses on insect susceptibility to parasites, predators, and diseases. Symp Am Chem Soc 208:37–55
Smith FA, Raven JA (1979) Intracellular pH and its regulation. Ann Rev Plant Phys 30:289–311
Swain T (1977) Secondary compounds as protection agents. Ann Rev Plant Physiol 28:479–501
Turunen S (1979) Lipid digestion and uptake in insects. Comp Biochem Physiol 63A:455–460
USDA (1981) The Gypsy Moth: Research Toward Integrated Pest Management. Doane CC, McManus ML (eds) USDA FS Tech Bull 1584
Van Sumere CF, Albrecht J, Dedonder A, DePooter H, Pe I (1975) Plant proteins and phenolics. In: Harborne JB, Van Sumere CF (eds) The Chemistry and Biochemistry of Plant Proteins, Academic Press, NY, USA, pp 211–264
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schultz, J.C., Lechowicz, M.J. Hostplant, larval age, and feeding behavior influence midgut pH in the gypsy moth (Lymantria dispar). Oecologia 71, 133–137 (1986). https://doi.org/10.1007/BF00377332
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00377332