Abstract
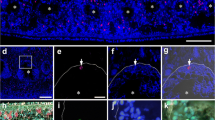
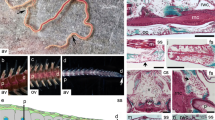
Ultrastructure of the resorption of integumentary tissues (ligaments, muscles, fibrous tissue, nerves, and skeleton) and the synthesis of collagen is described for the first time in echinoderms. Resorption is cell-mediated. Phagocytic cells are characterized by Golgi-derived putative primary lysosomes. Numerous secondary lysosomes and residual bodies occur in the bodies and processes of phagocytic cells. They engulf whole muscle cells and nerve fibres, as well as collagen fibril segments that exceed 1.5 μm in length. Skeletoclastic cells resemble vertebrate osteoclasts, showing a ruffled border, lysosomes, and numerous mitochondria. They surround trabeculae with thick processes to delimit a tubular resorption site. Collagen synthesis occurs in the space formerly occupied by resorbed tissues. Synthesis is performed by fibroblastic cells containing organelles typical of vertebrate fibroblasts, namely distended cisternae of rough endoplasmic reticulum, Golgi cisternae with distended edges, and procollagen granules. Procollagen granules are apparently exocytosed directly to the extracellular matrix. Evidence indicates that resorbing (phagocytic and skeletoclastic) cells and fibroblastic cells may belong to a common phagocyte lineage. These cells share the ability to form elaborate processes and to become syncytial, and their nuclei exhibit iron-containing crystals.
Similar content being viewed by others
References
Bachmann S, Goldschmid A (1978) Fine structure of the axial complex of Sphaerechinus granularis (Lam.) (Echinodermata: Echinoidea). Cell Tissue Res 193:107–123
Beig D, Da Cruz-Landim C (1975) Sperm reabsorption in sea urchin (Echinometra locunter). Ciência Cultura 27:221–228
Birk DE, Trelstad RL (1985) Metazoan mesenchyme partitions the extracellular space during matrix morphogenesis. In: Bairati A, Garrone R (eds) Biology of invertebrate and lower vertebrate collagens. Plenum Press, New York, pp 103–114
Bureau F, Dubois P, Ghyoot M, Jangoux M (1991) Skeleton resorption in echinoderms: Regression of pedicellarial stalks in Sphaerechinus granularis (Echinodermata). Zoomorphology 110:217–226
Campbell AC (1983) Form and function of pedicellariae: a review. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 1. A.A. Balkema, Rotterdam, pp 139–167
Candia-Carnevali MD, Lucca E, Bonasoro F (1993) Mechanisms of arm regeneration in the feather star Antedon mediterranea: healing of wound and early stages of development. J Exp Zool 267:299–317
Chia F-S (1970) Histology of the globiferous pedicellariae of Psammechinus miliaris (Echinodermata, Echinoidea). J Zool (London) 160:9–16
Chia F-S, Burke RD (1978) Echinoderm metamorphosis: fate of larval structures. In: Chia F-S, Rice ME (eds) Settlement and metamorphosis of marine invertebrate larvae. Elsevier, New York, pp 219–234
Dubois P, Chen C-P (1989) Calcification in echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 3. A.A. Balkema, Rotterdam, pp 109–178
Dubois P, Bureau F, Ghyoot M (1994) Induction of tissue resorption in globiferous pedicellariae of the echinoid Sphaerechinus granularis. In: David B, Guille A, Féral J-P, Roux M (eds) Echinoderms through time. A.A. Balkema, Rotterdam, pp 643–646
Féral J-P (1988) Wound healing after arm amputation in Sepia officinalis (Cephalopoda: Sepioidea). J Invert Pathol 52:380–388
Ferrand J-G (1983) Ultrastructural analysis of oocyte lysis and phagocytic activity in gonals of Asterina gibbosa P. (Echinodermata: Asteroidea). Internat J Invert Reprod 6:21–29
Ganter P, Jollès G (1970) Histochimie normale et pathologique, vol 2. Gauthiers-Villars, Paris
Ghyoot M, Jangoux M (1988) The fine structure of the globiferous pedicellariae of Sphaerechinus granularis (Echinoidea): innervation of the stalk. In: Burke RD, Mladenov PV, Lambert P, Parsley RL (eds) Echinoderm biology. A.A. Balkema, Rotterdam, pp 713–717
Ghyoot M, Dubois P, Jangoux M (1990) Ultrastructure and presumed origin of the phagocytic cells involved in the regression of headless stalks of globiferous pedicellariae in the echinoid Sphaerechinus granularis (Echinodermata). In: De Ridder C, Dubois P, Lahaye M-C, Jangoux M (eds) Echinoderm research. A.A. Balkema: Rotterdam, pp 239–245
Grimmer JC, Holland ND, Messing CG (1984) Fine structure of the stalk of the bourgeticrinid sea lily Democrinus conifer (Echinodermata: Crinoidea). Mar Biol (Berlin) 81:163–176
Heatflied BM, Travis DF (1975a) Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus. I. Cell types without spherules. J Morphol 145:13–50
Heatfield BM, Travis DF (1975b) Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus. II. Cell types with spherules. J Morphol 145:51–72
Hilgers H, Splechtna H (1982) Zur Steuerung der Ablös ung Giftpedizellarien bei Sphaerechinus granularis (Lam.) und Paracentrotus lividus (Lam.) (Echinodermata, Echinoidea). Zool Jahrb Anat 107:442–457
Holland LZ, Holland ND (1975) The fine structure of epidermal glands of regenerating and mature globiferous pedicellariae of a sea urchin (Lytechinus pictus). Tissue Cell 7:723–737
Holland ND, Grimmer JC (1981) Fine structure of the ciari and a possible mechanism for their motility in stalkless crinoids (Echinodermata). Cell Tissue Res 214:207–217
Hunt TK, Banda MJ, Silver IA (1985) Cell interactions in posttraumatic fibrosis. Ciba Found Symp 114:127–149
Jensen M (1966) The response of two sea-urchins to the sea-star Marthasterias glacialis (L.) and other stimuli. Ophelia 3:209–219
Märkel K, Röser U (1983a) Calcite resorption in the spine of the echinoid Eucidaris tribuloides. Zoomorphology 103:43–58
Märkel K, Röser U (1983b) The spine tissues in the echinoid Eucidaris tribuloides. Zoomorphology 103:25–41
Märkel K, Röser U (1985) Comparative morphology of echinoderm calcified tissues: histology and ultrastructure of ophiuroid scales (Echinodermata, Ophiuroida). Zoomorphology 105:197–207
Märkel K, Röser U, Mackenstedt U, Klostermann M (1986) Ultrastructural investigations of matrix-mediated biomineralization in echinoids (Echinodermata, Echinoidea). Zoomorphology 106:232–243
Marks SC Jr, Popoff SN (1988) Bone cell biology: the regulation of development, structure, and function in the skeleton. Am J Anat 183:1–44
Masuda R, Dan JC (1977) Studies on the annual reproductive cycle of the sea urchin and the acid phosphotase activity of relict ova. Biol Bull, Woods Hole 153:577–590
Menton DN, Eisen AZ (1973) Cutaneous wound healing in the sea cucumber Thyone briareus. J Morphol 141:185–204
Mladenov PV, Bisgrove B, Asotra S, Burke RD (1989) Mechanisms of arm-tip regeneration in the sea star, Leptasterias hexactis. Roux's Arch Develop Biol 198:19–28
Parakkal PE (1969) Involvement of macrophages in collagen resorption. J Cell Biol 41:345–354
Pérès JM (1950) Recherches sur les pedicellaires glandulaires de Sphaerechinus granularis (Lamarck). Arch Zool Exp Gén 86:118–136
Ralphs JR, Brown RA (1986) A characteristic secretory organelle in early osteoblastic cells: a fibrillar inclusion body. In: Ali SY (ed) Cell mediated calcification and matrix vesicles. Elsevier, Amsterdam, pp 167–172
Richardson KC, Jarret L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Sire J-Y (1985) The deep scleroblast of the regenerating teleost scale: a model of cell producing a collagenic plywood. In: Bairati A, Garrone R (eds) Biology of invertebrate and lower vertebrate collagens. Plenum Press, New York, pp 465–470
Sire J-Y, Huysseune A, Meunier FJ (1990) Osteoclasts in teleost fish: light- and electron-microscopical observations. Cell Tissue Res 260:85–94
Ten Cate AR, Deporter DA (1975) The degradative role of the fibroblast in the remodelling and turnover of collagen in soft connective tissue. Anat Rec 182:1–14
Trelstad RL (1971) Vacuoles in the embryonic chick corneal epithelium, an epithelium which produces collagen. J Cell Biol 48:689–694
Trelstad RL, Birk DE (1985) The fibroblast in morphogenesis and fibrosis: cell topography and surface-related functions. Ciba Found Symp 114:4–19
Trelstad RL, Hayashi K (1979) Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Develop Biol 71:228–242
Vaes G (1988) Cellular biology and biochemical mechanism of bone resorption. Clin Orthoped 231:239–271
Vanden Bossche JP, Jangoux M (1976) Epithelial origin of starfish coelomocytes. Nature 261:227–228
Walker CW (1979) Ultrastructure of the somatic portion of the gonads in asteroids, with emphasis on flagellated-collar cells and nutrient transport. J Morphol 162:127–162
Walker CW (1980) Spermatogenic columns, somatic cells, and the microenvironment of germinal cells in the testes of asteroids. J Morphol 166:81–107
Weber W, Grosmann M (1977) Ultrastructure of the basiepithelial nerve plexus of the sea urchin Centrostephanus longispinus. Cell Tissue Res 175:551–562
Weinstock M, Leblond CP (1974) Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after 3H-proline administration. J Cell Biol 60:92–127
Author information
Authors and Affiliations
Additional information
This project was supported by Contract 2.4527.89 from the Fonds de la Recherche Fondamentale Collective (Belgium). P.D. is a Research Associate of the National Fund for Scientific Research (Belgium). Contribution of the Centre Interuniversitaire de Biologie Marine.
Rights and permissions
About this article
Cite this article
Dubois, P., Ghyoot, M. Integumentary resorption and collagen synthesis during regression of headless pedicellariae in Sphaerechinus granularis (Echinodermata: Echinoidea). Cell Tissue Res 282, 297–309 (1995). https://doi.org/10.1007/BF00319120
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00319120