Abstract
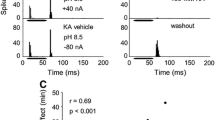
The effect of microiontophoretically applied γ-aminobutyric acid (GABA) and its agonists and antagonists on the response pattern of single units in the ventral cochlear nucleus (VCN) of the rat was examined in order to study GABA's physiological function in auditory processing. The effects of the drugs were judged by changes of spontaneous and sound-evoked activity in peristimulus-time histograms (PSTHs) of at least 20 consecutive presentations of acoustic stimuli. GABA inhibited the discharge activity of the majority of neurons. All response types found in the VCN except onset-I responders were sensitive to GABA. The GABAergic inhibition is most probably mediated by GABAA receptors, since the GABAA-receptor agonist muscimol, but not the GABAB-receptor agonist baclofen, mimicked the effect of GABA. The GABAA-receptor antagonists, bicuculline and picrotoxin, had an excitatory effect on the neurons' spontaneous activity, suggesting a tonic endogeneous release of GABA which exerts a permanent inhibition on VCN neurons. Although inhibitory, iontophoresis of GABA emphasized the response to stimulus onset in the PSTHs by means of a stronger inhibition of spontaneous activity. When using iontophoretical currents which did not suppress the neuronal activity completely, a strong inhibition of spontaneous activity was accompanied by only a small inhibition of tone-evoked activity. Under these conditions, the response to tone onset was frequently not inhibited at all. Therefore, GABA's physiological function is possibly to improve the contrast between transient acoustic signals and ongoing background activity. In order to test this hypothesis, the test tone was masked by continuous background noise. Indeed, GABA reduced the noise-evoked discharge more than the toneevoked discharge, leaving the onset peak in the PSTHs almost unchanged. Thus, GABAergic input improves the signal-to-noise ratio for acoustic transients in VCN neurons. Our data suggest that a functional role of GABA in the VCN is to act as a transmitter within a descending inhibitory feedback loop of the auditory brainstem which serves to improve the transmission of relevant acoustic signals in constant background noise.
Similar content being viewed by others
References
Aams JC, Mugnaini E (1987) Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. J Comp Neurol 262:375–401
Altschuler RA, Betz H, Parakkal MH, Reeks KA, Wenthold RJ (1986) Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res 369:316–320
Altschuler RA, Juiz J, Shore S, Bledsoe S, Helfert R, Wenthold R (1993) Inhibitory amino acid synapses and pathways in the ventral cochlear nucleus. In: Merchan MA, Juiz JM, Godfrey DA, Mugnaini E (eds) The mammalian cochlear nuclei. Plenum Press, New York, pp 211–224
Cant NB (1991) Projections to the lateral and medial superior olivary nuclei from spherical and globular bushy cells of the anteroventral cochlear nucelus. In: Altschuler RA et al (ed) Neurobiology of hearing: the central auditory system. Raven, New York, pp 99–119
Cant NB, Gaston KC (1982) Pathways connecting the right and left cochlear nuclei. J Comp Neurol 212:313–326
Caspary DM, Havey DC, Faingold CL (1979) Effects of microiontophoretically applied glycine and GABA on neuronal response patterns in the cochlear nuclei. Brain Res 172:179–185
Caspary DM, Rybak LP, Faingold CL (1984) Baclofen reduces tone-evoked activity of cochlear nucleus neurons. Hear Res 13:113–122
Caspary DM, Rybak LP, Faingold CL (1985) The effects of inhibitory and excitatory ammo-acid neurotransmitters on the response properties of brainstem auditory neurons. In: Drescher DG (eds) Auditory bochemistry. Thomas, Springfield, pp 198–225
Caspary DM, Pazara KE, Kössl M, Faingold CL (1987) Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res 417:273–282
Colombo J, Frisina RD (1992) Neural processing by the cochlear nucleus. Soc Neurosci Abstr 18:150
Ebert U, Koch M (1992) Glutamate receptors mediate acoustic input to the reticular formation. Neuroreport 3:429–432
Ebert U, Ostwald J (1991) Modulation of auditory responses by GABA and noradrenaline in the cochlear nucleus of the rat. In: Elsner N, Penzlin H (eds) Synapse — transmission — modulation. Thieme, Stuttgart, p 119
Evans EF, Nelson PG (1973) The responses of single neurons in the cochlear nucleus of the cat as a function of their location and anaesthetic state. Exp Brain Res 17:402–427
Evans EF, Zhao W (1993) Varieties of inhibition in the processing and control of processing in the mammalian cochlear nucleus. Prog Brain Res 97:117–126
Friauf E, Ostwald J (1988) Divergent projections of physiologically characterized rat ventral cochlear nucleus as shown by intracellular injection of horseradish peroxidase. Exp Brain Res 73:263–284
Glendenning KK, Baker BN (1988) Neuroanatomical distribution of receptors for three potential inhibitory neurotransmitters in the brainstem auditory nuclei of the cat. J Comp Neurol 275:288–308
Greenwood DD, Goldberg JM (1970) Responses of neurones in the cochlear nuclei to variations in noise bandwidth and to tone-noise combinations. J Acoust Soc Am 47:1022–1040
Irvine DRF (1986) The auditory brainstem. Springer, Berlin Heidelberg New York, pp 40–78
Juiz JM, Helfert RH, Wenthold RJ, De Blas AL, Altschuler RA (1989) Immunocytochemical localization of the GABAA/benzodiazepine receptor in the guinea pig cochlear nucleus: evidence for receptor localization heterogeneity. Brain Res 504:173–179
Juiz JM, Albin RL, Helfert RH, Altschuler RA (1994) Distribution of GABAA and GABAB binding sites in the cochlear nucleus of the guinea pig. Brain Res 639:193–201
Kandler K, Herbert H (1991) Auditory projections from the cochlear nucleus to pontine and mesencephalic reticular nuclei in the rat. Brain Res 562:230–242
Koch M, Lingenhöhl K, Pilz PKD (1992) Loss of the acoustic startle response following neurotoxic lesions of the caudal pontine reticular formation — possible role of giant neurons. Neuroscience 49:617–625
Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J (1992) An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol (Berl) 186:443–465
Kössl M, Vater M (1989) Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci 9:4169–4178
Lingenhöhl K, Friauf E (1992) Giant neurons in the caudal pontine reticular formation receive short latency acoustic input an intracellular recording and HRP study in the rat. J Comp Neurol 325:473–492
Lingenhöhl K, Friauf E (1994) Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci 14:1176–1194
Martin MR, Dickson JW, Fex J (1982) Bicuculline, strychnine and depressant amino acid responses in the anteroventral cochlear nucleus of the cat. Neuropharmacology 21:201–207
Mesulam MM (1982) Tracing neural connections with horseradish peroxidase. Wiley, Chichester
Moore JA, Appenteng K (1990) Contrasting effects of urethane and pentobarbitone anaesthesia on the electrical properties of rat jaw-elevator motoneurones. Brain Res 523:139–142
Moore JK, Moore RY (1987) Glutamic acid decarboxylase-like mmunoreactivity in brainstem auditory nuclei of the rat. J Comp Neurol 260:157–174
Oertel D (1983) Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J Neurosci 3:2043–2053
Oertel D (1991) The role of intrinsic neuronal properties in the encoding of auditory information in the cochlear nuclei. Curr Opin Neurobiol 1:221–228
Ostapoff E-M, Morest DK (1991) Synaptic organization of globular bushy cells in the ventral cochlear nucleus of the cat: a quantitative study. J Comp Neurol 314:598–613
Ostapoff E-M, Morest DK, Potashner SJ (1990) Uptake and retrograde transport of [3H]GABA from the cochlear nucleus to the superior olive in the guinea pig. J Chem Neuroanat 3:285–295
Palmer AR, Evans EF (1982) Intensity coding in the auditory periphery of the cat: responses of cochlear nerve and cochlear nucleus neurons to signals in the presence of bandstop masking noise. Hear Res 7:305–323
Palombi PS, Caspary DM (1992) GABAA receptor antagonist bicuculline alters response properties of posteroventral cochlear nucleus neurons. J Neurophysiol 67:738–746
Peyret D, Geffard M, Aran J-M (1986) GABA immunoreactivity in the primary nuclei of the auditory central nervous system. Hear Res 23:115–121
Pfeiffer RR (1966) Classification of response patterns of spike discharges for units in the cochlear nucleus: tone-burst stimulation. Exp Brain Res 1:220–235
Rhode WS (1991) Physiological-morphological properties of the cochlear nucleus. In: Altschuler RA, et al (eds) Neurobiology of hearing: the central auditory system. Raven, New York, pp 47–77
Rhode WS, Smith PH (1986) Encoding time and intensity in the ventral cochlear nucleus of the cat. J Neurophysiol 56:261–286
Roberts RC, Ribak CE (1987) GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol 258:267–280
Saint Marie RL, Morest DK, Brandon CJ (1989) The form and distribution of GABAergic synapses on the principal cell types of the ventral cochlear nucleus of the cat. Hear Res 42:97–112
Shannon-Hartman S, Godfrey DA, Dunn JD, Godfrey TG, Farms WB (1993) Effects of large brain stem lesions on amino acid chemistry in the rat cochlear nucleus. Soc Neurosci Abstr 19:533
Smith PH, Joris PX, Carney LH, Yin TC (1991) Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol 304:387–407
Spangler KM, Cant NB, Henkel CK, Farley GR, Warr WB (1987) Descending projections from the superior olivary complex to the cochlear nucleus of the cat. J Comp Neurol 259:452–465
Spirou GA, Young ED (1991) Organization of dorsal cochlear nucleus type IV unit response maps and their relationship to activation by bandlimited noise. J Neurophysiol 66:1750–1768
Thompson AM, Thompson GC (1991) Projections from the posteroventral cochlear nucleus to the superior olivary complex in guinea pig: light and EM observations with the PHA-L method. J Comp Neurol 311:495–508
Voigt HF, Young ED (1980) Evidence of inhibitory interactions between neurons in the dorsal cochlear nucleus. J Neurophysiol 44:76–96
Voigt HF, Young ED (1990) Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol 64:1590–1610
Walsh EJ, McGee J, Fitzakerley JL (1990) GABA actions within the caudal cochlear nucleus of developing kittens. J Neurophysiol 64:961–977
Wenthold RJ (1987) Evidence for a glycinergic pathway connecting the two cochlear nuclei: an immunocytochemical and retrograde transport study. Brain Res 415:183–187
Wenthold RJ (1991) Neurotransmitters of brainstem auditory nuclei. In: Altschuler RA et al (eds) Neurobiology of hearing: the central auditory system. Raven, New York, pp 121–139
Wenthold RJ, Zempel JM, Parakkal MH, Reeks KA, Altschuler RA (1986) Immunocytochemical localization of GABA in the cochlear nucleus of the guinea pig. Brain Res 380:7–18
Wenthold RJ, Huie D, Altschuler RA (1987) Glycine immunoreactivity localized in the cochlear nucleus and superior olivary complex. Neurosci 22:897–912
Wenthold RJ, Parakkal MH, Oberdorfer MD, Altschuler RA (1988) Glycine receptor immunoreactivity in the ventral cochlear nucleus of the guinea pig. J Comp Neurol 276:423–435
Wickesberg RE, Oertel D (1990) Delayed, frequency-specific inhibition in the cochlear nuclei of mice: a mechanism for monaural echo suppression. J Neurosci 10:1762–1768
Wu SH, Oertel D (1984) Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci 4:1577–1588
Wu SH, Oertel D (1986) Inhibitory circuitry in the ventral cochlear nucleus is probably mediated by glycine. J Neurosci 6:2691–2706
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ebert, U., Ostwald, J. GABA can improve acoustic contrast in the rat ventral cochlear nucleus. Exp Brain Res 104, 310–322 (1995). https://doi.org/10.1007/BF00242016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00242016