Abstract
Background
Clopidogrel selectively inhibits platelet aggregation. Clopidogrel bisulfate (Plavix®) was first developed for atherothrombosis prevention and is commonly prescribed for this indication. A new clopidogrel formulation, clopidogrel besylate (KOVIX®), has recently been developed.
Objective
This study was designed to compare the multiple-dose pharmacokinetics/pharmacodynamics and tolerability of clopidogrel besylate with those of clopidogrel bisulfate in 40 healthy male subjects.
Methods
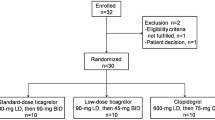
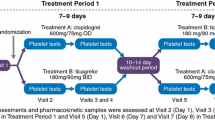
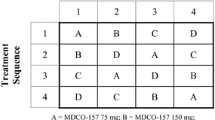
This was an open-label, randomized-sequence, multiple-dose, two-period, two-treatment crossover study. The subjects were randomly assigned to a sequence group that received two treatments: clopidogrel besylate 75 mg followed by clopidogrel bisulfate 75 mg, or vice versa. The subjects received a 300-mg loading dose on day 1 followed by 75 mg daily for the next 4 days. Serial blood samples were collected to determine the concentrations of clopidogrel and its carboxylic acid metabolite, SR26334. Platelet aggregation and bleeding times were measured. Tolerability was evaluated throughout the study.
Results
The clopidogrel plasma concentration–time profiles of the formulations were similar. The measured pharmacokinetic parameters did not differ significantly between the clopidogrel besylate and clopidogrel bisulfate groups. The geometric mean ratios of the clopidogrel besylate group to the clopidogrel bisulfate group with respect to the maximum plasma concentration (Cmax) and the area under the concentration–time curve (AUC) from time zero to the time of last measurable concentration (AUClast) were 0.96 (90 % confidence interval [CI] 0.82, 1.12) and 0.95 (0.81, 1.11), respectively. Moreover, the pharmacokinetic parameters of SR26334 did not differ significantly between the two treatment groups. Furthermore, the areas under the platelet aggregation inhibition-time curves (AUIC) and the maximum inhibitory effects (Imax) did not differ significantly between the two groups. The geometric mean ratios (clopidogrel besylate to clopidogrel bisulfate) were 1.01 (90 % CI 0.95, 1.08) for the Imax and 0.98 (0.89, 1.07) for the AUIC. Both formulations were well tolerated and exhibited comparable safety profiles.
Conclusion
This study demonstrated that the pharmacokinetic/pharmacodynamic profiles of clopidogrel besylate were not significantly different from those of clopidogrel bisulfate. Both formulations were well tolerated in healthy subjects.



Similar content being viewed by others
References
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Nityanand S, Pande I, Bajpai VK, Singh L, Chandra M, Singh BN. Platelets in essential hypertension. Thromb Res. 1993;72(5):447–54.
Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106(22):2800–5.
Broijersen A, Hamsten A, Eriksson M, Angelin B, Hjemdahl P. Platelet activity in vivo in hyperlipoproteinemia–importance of combined hyperlipidemia. Thromb Haemost. 1998;79(2):268–75.
Hioki H, Aoki N, Kawano K, Homori M, Hasumura Y, Yasumura T, et al. Acute effects of cigarette smoking on platelet-dependent thrombin generation. Eur Heart J. 2001;22(1):56–61.
Davi G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322(25):1769–74.
Cyrus T, Sung S, Zhao L, Funk CD, Tang S, Pratico D. Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2002;106(10):1282–7.
Collaborative overview of randomised trials of antiplatelet therapy–I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81–106.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329–39.
Savi P, Labouret C, Delesque N, Guette F, Lupker J, Herbert JM. P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun. 2001;283(2):379–83.
Bristol-Myers Squibb / Sanofi Pharmaceuticals Partnership. Plavix (clopidogrel bisulfate) prescribing information. http://products.sanofi.us/plavix/plavix.html. Accessed January 3, 2012.
Jarvis B, Simpson K. Clopidogrel: a review of its use in the prevention of atherothrombosis. Drugs. 2000;60(2):347–77.
Pfizer. Norvasc (amlodipine besylate) prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=562. Accessed January 3, 2012.
Lyseng-Williamson KA. Oral bepotastine: in allergic disorders. Drugs. 2010;70(12):1579–91.
Bryson HM, Faulds D. Cisatracurium besilate. A review of its pharmacology and clinical potential in anaesthetic practice. Drugs. 1997;53(5):848–66.
Jung TR, Hwang SW, Lee JU, Cho MJ, Kim Y, Yoo TW. Comparative consistency between obesity determination standards using Body Mass Index and Ideal body weight. J Korean Acad Fam Med. 2001;22:1765–71.
Jernberg T, Payne CD, Winters KJ, Darstein C, Brandt JT, Jakubowski JA, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27(10):1166–73.
Kim KA, Park PW, Hong SJ, Park JY. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008;84(2):236–42.
Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921–86.
Kim SD, Kang W, Lee HW, Park DJ, Ahn JH, Kim MJ, et al. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther. 2009;31(4):793–803.
Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. 2011;89(1):65–74.
Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):25–8.
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38(1):92–9.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–62.
Klinkhardt U, Kirchmaier CM, Westrup D, Graff J, Mahnel R, Breddin HK, et al. Ex vivo–in vitro interaction between aspirin, clopidogrel, and the glycoprotein IIb/IIIa inhibitors abciximab and SR121566A. Clin Pharmacol Ther. 2000;67(3):305–13.
Acknowledgments
This study was sponsored by Kolon Pharmaceuticals, Seoul, Korea. The authors have indicated that they have no other conflicts of interest relevant to the content of this article. All of the co-authors were involved in the writing of the manuscript. The authors thank Ms SungHee Han (Clinical Trials Center at Seoul National University Hospital) for performing the platelet aggregation tests in this study. The manuscript was edited for English language by American Journal Experts (http://www.journalexperts.com/).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, BH., Kim, JR., Lim, K.S. et al. Comparative Pharmacokinetics/Pharmacodynamics of Clopidogrel Besylate and Clopidogrel Bisulfate in Healthy Korean Subjects. Clin Drug Investig 32, 817–826 (2012). https://doi.org/10.1007/s40261-012-0007-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-012-0007-3