Abstract
Streptococcus pyogenes causes severe, invasive infections such as the sequelae associated with acute rheumatic fever, rheumatic heart disease, acute glomerulonephritis, uncomplicated pharyngitis, and pyoderma. Efforts to produce a vaccine against S. pyogenes began several decades ago, and different models have been proposed. We have developed a vaccine candidate peptide, StreptInCor, comprising 55 amino acid residues of the C-terminal portion of the M protein and encompassing both the T- and B-cell protective epitopes. The present article summarizes data from the previous 5 years during which we tested the immunogenicity and safety of StreptInCor in different animal models. We showed that StreptInCor overlapping peptides induced cellular and humoral immune responses of individuals bearing different HLA class II molecules. These results are consistent with peptides that have a universal vaccine epitope. The tridimensional molecular structure of StreptInCor was elucidated by nuclear magnetic resonance spectroscopy, which showed that its structure is composed of two microdomains linked by an 18-residue α-helix. Additionally, we comprehensively evaluated the structural stability of the StreptInCor peptide in different physicochemical conditions using circular dichroism. Additional experiments were performed with inbred, outbred, and HLA class II transgenic mice. Analysis of several organs of these mice showed neither deleterious nor autoimmune reactions even after a long period of vaccination, indicating that the StreptInCor candidate peptide could be considered as an immunogenic and safe vaccine.
Similar content being viewed by others
1 Introduction
Group A streptococci (GAS) diseases remain a major public health problem in developing countries, reaching 600 million cases per year and thus constituting an important cause of morbidity and mortality. GAS infections can lead to severe invasive diseases including pharyngitis and pyoderma and to autoimmune post-streptococcal sequelae, such as rheumatic fever (RF) and glomerulonephritis [1]. At least 517,000 deaths each year are estimated to result from severe GAS infections, and its prevalence is at least 18.1 million cases, with 1.78 million new cases arising each year. The burden of invasive GAS diseases is surprisingly high, with at least 663,000 new cases and 163,000 deaths each year. In addition, there are more than 111 million prevalent cases of GAS pyoderma and over 616 million incidental cases of GAS-induced pharyngitis each year. Rheumatic heart disease (RHD) is the most serious outcome of RF, leading to progressive and permanent lesions of the heart valves. The worldwide incidence of RHD is at least 15.6 million cases with 233,000 deaths each year [1]. In Brazil, heart-valve damage due to RF is responsible for approximately 90 % of heart surgeries in children, resulting in high costs to the Brazilian health system [2].
Considering this large number of GAS infections and the potential sequelae, control strategies such as the development of an anti-streptococcal vaccine that is able to prevent S. pyogenes infection and colonization are extremely important.
2 Streptococcus pyogenes Vaccines
Previously, to prevent infections caused by S. pyogenes, different approaches to construct vaccines with distinct compositions were adopted. Some models are based on developing vaccines targeting the M protein (N- and C-terminal portions), the major antigen of S. pyogenes. Other vaccines are based on using different bacterial antigens, such as the streptococcal C5a peptidase, group A streptococcal carbohydrate, streptococcal fibronectin-binding proteins, cysteine proteases, streptococcal pyrogenic exotoxins, and streptococcal pili [3–12].
In this article, we briefly present our approach to the development of a vaccine against S. pyogenes. We initially tested 79 overlapping candidate peptides using a large panel of human sera and T cells from healthy individuals and RF/RHD patients. These peptides covered a range of 100 amino acids and corresponded to the C-terminal portion of the streptococcal M5 protein. An immunogenic peptide epitope consisting of 55 amino acid residues was identified from this study and named StreptInCor (medical identification) [9]. This immunogenic epitope needs to be recognized and then processed by macrophages, leading to the generation of small peptides of approximately 15 amino acid residues. These peptides are subsequently presented by HLA class II molecules of the macrophages to T lymphocytes via the T-cell receptor (TCR) (Fig. 1). HLA class II molecules are expressed on the surface of antigen-presenting cells such as macrophages, dendritic cells and B lymphocytes (Fig. 1). As diverse peptides from the StreptInCor will be generated, they will bind with different HLA class II molecules as previously described [13].
Antigen processing and presentation. Antigen-presenting cells (APCs) including monocytes, macrophages, dendritic cells, and B lymphocytes, phagocytose and process the StreptInCor peptide. Processed peptides are presented by different HLA-DR molecules to T lymphocytes through their T-cell receptors (TCRs)
The StreptInCor peptide epitope has universal immunogenic characteristics because its amino acid sequence can be recognized in the context of any class II major histocompatibility complex (MHC) molecule, which means that any individual will be able to develop an immune response after vaccination [13].
Molecular-based methods have been adopted for structural analyses of peptide-based vaccines. This subject has attracted special attention because the chemical and structural integrity of vaccine components can potentially interfere with their immunogenic properties. Nuclear magnetic resonance analysis of the StreptInCor candidate peptide showed amino acid residues in both the α-helical and disordered conformations (Fig. 2). Interestingly, this spatial conformation is central for recognition of the StreptInCor peptide by both T and B lymphocytes and, consequently, for the specific and strong immune responses mediated by both T cells and antibodies. Hence, the three-dimensional structural features of the StreptInCor peptide epitope are favorable for both MHC class II and TCR peptide interactions. These interactions result in the broad recognition of the vaccine peptide by individuals bearing any MHC class II molecule with consequent activation of both T and B cells, which undergo further maturation from effector to memory cells [13].
Nuclear magnetic resonance structure of StreptInCor peptide. T epitopes (residues 1 to 22) and B epitopes (residues 31 to 55) linked by eight residues are shown in the model. These peptides correspond with residues 253 to 309 of the native Streptococcus pyogenes M5 protein [6]
For a vaccine to induce an effective immune response against a pathogen such as S. pyogenes, both the humoral and cellular immune responses are required. Furthermore, during the process of vaccine development, other biologic mechanisms and molecular criteria, such as structure determination and physicochemical properties, should be considered. Factors affecting vaccine inactivation and stability have been studied by spectroscopic and calorimetric techniques such as dynamic light scattering, fluorescence, circular dichroism, and differential scanning calorimetry [14]. Peptides with high hydrophobic amino acid content tend to pack together, forming disordered complexes not easily identifiable as visible precipitates. Despite being soluble, these aggregates can interfere with immunotherapeutic administration of the vaccine and disrupt the adequate addressing of the active principle. In this context, the detection of soluble aggregates is particularly important and can be assessed by right-angle light scattering. Additionally, the chemical and structural stability of vaccines are intimately related to both the cost and efficacy of immunization programs, particularly in some regions of the world where the distribution of cold chains might be unavailable. In our recent work, we have shown that the folding and refolding mechanism of StreptInCor peptide proceeds as a reversible process [13]. These studies showed that the StreptInCor peptide comprises several necessary qualities required for a vaccine, as its structure reverts to the original state over a wide range of temperatures, pH, and concentrations of a chaotropic agent. Table 1 summarizes some of the physicochemical proprieties of the StreptInCor peptide. Taken together, these data can be interpreted as the requirement for less stringent conditions for excipients, pH, and temperature during processing, transport, and storage.
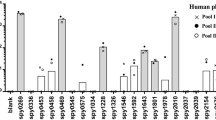
Previously, we reported the ability of two pools of sera from healthy individuals with positive and negative anti-streptolysin O. Both pools reacted to the selected B-cell epitope to inhibit adhesion and invasion of HEp2 cells by several strains of S. pyogenes. A high percentage of inhibition of adhesion/invasion was detected against several strains: M5 and M6 (99 %), M44/61 (98 %), M87 (97 %), M1 (90 %), M71 and M22 (70 %) [9].
StreptInCor was also assayed in experimental models of inbred and outbred mice lineages in the presence of complete Freund’s adjuvant (CFA) or alum. High titers of immunoglobulin (Ig) G1 and IgG2a were induced after immunization of mice with the StreptInCor peptide. In addition, these IgG antibodies were able to recognize the StreptInCor epitope in a heterologous, recombinant streptococcal M1 protein that shares 74 % of identical residues with the M5 C-terminal portion. This result indicates that the selected region of the StreptInCor peptide has a potential protective effect [10]. Immunization of BALB/c mice was performed with 100 μg of the StreptInCor peptide vaccine in a conventional Freund’s adjuvant and a new adjuvant (AFCo1) that induces mucosal immune responses. AFCo1 is generated by calcium precipitation of a proteoliposome that is derived from the outer membrane of Neisseria meningitides B. The co-administration of the StreptInCor peptide epitope and the AFCo1 adjuvant induced both mucosal (IgA) and systemic (IgG) antibodies, as is normally observed in polarized Th1-mediated immune responses [10]. This adjuvant has been used for several experimental immunization assays and may be a promising adjuvant for future use in humans [15].
Additionally, we conducted experimental assays to determine the safety of the StreptInCor vaccine epitope. The safety of the vaccine was assessed using DR2, DR4, DQ6, and DQ8 HLA class II transgenic mice [16]. These transgenic mice do not express murine H2 molecules and are an important tool for studying the relationship of HLA class II molecules and the development of autoimmune diseases. In addition, this model could be useful for studying the immune response to vaccines, particularly against S. pyogenes, to control possible autoimmune reactions in some organs, similar to those observed in RF and RHD. The longevity of immune responses in these transgenic models was also evaluated against StreptInCor adsorbed onto alum by following the animals for 1 year after the immunization. Histopathological examination of joints, brain, heart tissue (mitral, aortic, and tricuspid valves), kidneys, spleen, and liver from these animals did not show any alterations. These findings were demonstrated in cases of high titers of anti-StreptInCor IgG antibodies. Cross reactivity to human heart tissue was also not observed [16]. Subsequently, the pattern of spleen memory T cells of these immunized animals was evaluated after StreptInCor stimuli. The CD4+ and CD8+ cells were predominantly effector memory T cells (CD44+CD62L−). Only in the DQ8 lineage were the CD4+ cells predominantly central memory T cells (CD44+CD62L+). These data showed that StreptInCor immunization was able to induce a protective memory T-cell response against S. pyogenes. These data are summarized in Table 2. Taken together, these results are consistent with a robust, safe, and long-lasting immune response to StreptInCor without deleterious reactions.
Toxicological studies involving experiments with Wistar rats and mini-pigs are currently under way.
In conclusion, the data presented here reinforce the perspective that the development of an anti-S. pyogenes vaccine will increase the possibility of avoiding infections and hence reduce the number of new cases of RF and RHD.
References
Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94.
Barbosa PJB, Müller RE, Latado AL, Achutti AC, Ramos AIO, Weksler C, et al. (2009) Diretrizes Brasileiras para Diagnóstico, Tratamento e Prevenção da Febre Reumática da Sociedade Brasileira de Cardiologia, da Sociedade Brasileira de Pediatria e da Sociedade Brasileira de Reumatologia (Brazilian guidelines for the diagnosis, treatment and prevention of rheumatic fever). Arq Bras Cardiol. 93(3 Suppl 4):1–18.
Fischetti VA, Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. Identification of an immunodominant region. J Immunol. 1988;141(10):3592–9.
Olive C, Clair T, Yarwood P, Good MF. Protection of mice from group A streptococcal infection by intranasal immunisation with a peptide vaccine that contains a conserved M protein B cell epitope and lacks a T cell autoepitope. Vaccine. 2002;20(21–22):2816–25.
Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, Wasserman SS, Dale JB. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004;292(6):709–15.
Robinson JH, Atherton MC, Goodacre JA, Pinkney M, Weightman H, Kehoe MA. Mapping T-cell epitopes in group A streptococcal type 5 M protein. Infect Immun. 1991;59(12):4324–31.
Batzloff M, Yan H, Davies M, Hartas J, Good M. Preclinical evaluation of a vaccine based on conserved region of M protein that prevents group A streptococcal infection. Indian J Med Res. 2004;119(Suppl):104–7.
McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, Linden J, Fries LF, Vink PE, Dale JB. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41(8):1114–22.
Guilherme L, Faé KC, Higa F, Chaves L, Oshiro SE, Freschi de Barros S, Puschel C, Juliano MA, Tanaka AC, Spina G, Kalil J. Towards a vaccine against rheumatic fever. Clin Dev Immunol. 2006;13(2–4):125–32.
Guilherme L, Postol E, Freschi de Barros S, Higa F, Alencar R, Lastre M, Zayas C, Puschel CR, Silva WR, Sa-Rocha LC, Sa-Rocha VM, Pérez O, Kalil J. A vaccine against S. pyogenes: design and experimental immune response. Methods. 2009;49(4):316–21.
Pandey M, Batzloff MR, Good MF. Mechanism of protection induced by group A Streptococcus vaccine candidate J8-DT: contribution of B and T-cells towards protection. PLoS One. 2009;4(4):e5147.
Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis. 2009;22(6):544–52.
Guilherme L, Alba MP, Ferreira FM, Oshiro SE, Higa F, Patarroyo ME, Kalil J. Anti-group A streptococcal vaccine epitope: structure, stability, and its ability to interact with HLA class II molecules. J Biol Chem. 2011;286(9):6989–98.
Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal stability of vaccines. J Pharm Sci. 2003;92(2):218–31.
Acevedo R, Callicó A, del Campo J, González E, Cedré B, González L, Romeu B, Zayas C, Lastre M, Fernández S, Oliva R, García L, Pérez JL, Pérez O. Intranasal administration of proteoliposome-derived cochleates from Vibrio cholerae O1 induce mucosal and systemic immune responses in mice. Methods. 2009;49:309–15.
Guerino MT, Postol E, Demarchi LM, Martins CO, Mundel LR, Kalil J, Guilherme L. HLA class II transgenic mice develop a safe and long lasting immune response against StreptInCor, an anti-group A streptococcus vaccine candidate. Vaccine. 2011;29(46):8250–6.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guilherme, L., Ferreira, F.M., Köhler, K.F. et al. A Vaccine against Streptococcus pyogenes . Am J Cardiovasc Drugs 13, 1–4 (2013). https://doi.org/10.1007/s40256-013-0005-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-013-0005-8