Abstract
Purpose
Evaluation of the severity of pandemic influenza requires reliable estimates of mortality attributable to the seasonal influenza.
Methods
Excess age-specific mortality during periods of influenza activity was evaluated in Israel during the period 1999–2006 for three death categories. For each respiratory year, the lowest monthly moving average for the mortality rate was subtracted from each month in the period of influenza activity. Average mortality rates in years with minimal influenza activity were deducted from corresponding months to exclude winter mortality unrelated to influenza. The sums of these results were used as estimates of excess mortality rates.
Results
Overall excess mortality rates ranged from 7.7 to 36.1 per 100,000 for all causes, and from 4.4 to 24.4 per 100,000 for respiratory and circulatory causes. Influenza was associated with an average of 869 (range 280–1,516) deaths annually from respiratory and circulatory diseases during seasons with significant influenza activity. About 90% of the influenza-associated mortality from respiratory and circulatory diseases was in the age group 65+ years and about 1% in the age group <50 years. The age group <50 years accounted for an annual average of seven deaths from respiratory and circulatory diseases.
Conclusion
Annual mortality associated with seasonal influenza is highly variable. Under the age of 50 years, there is minimal seasonal influenza associated mortality. This information provides an important baseline for evaluating the severity of the A(H1N1) 2009 influenza pandemic, where persons under 50 years of age were often disproportionately represented.
Similar content being viewed by others
Introduction
The A(H1N1) 2009 influenza pandemic brought into focus the need for reliable estimates of mortality associated with seasonal influenza in order to be able to assess the potential impact of the pandemic. Several factors need to be accounted for when such estimates are being made. The diagnosis of influenza is usually based on typical symptoms and signs, rather than laboratory confirmation [1], and other viruses may ultimately be the causative agent, such as respiratory syncytial virus [1–4]. Furthermore, since death from influenza frequently results from secondary bacterial pneumonia or a general deterioration due to pre-existing cardio-respiratory diseases [1, 5–7], the preceding influenza-like-illness either is not suspected as an underlying cause of death or is simply not mentioned on the death notification. As a consequence, estimates of influenza-associated mortality have usually been made on the basis of excess mortality from all-causes, respiratory and circulatory diseases, or pneumonia and influenza [1, 2, 8–11]. The use of these surrogate measures can be confounded by excess mortality that tends to occur during the colder months of the year [12], in particular for deaths from respiratory and cardiovascular diseases, which may result from extreme climatic conditions or changes in temperature [13, 14].
In the USA, seasonal influenza-associated mortality rates, based on excess deaths due to respiratory and circulatory causes during periods of influenza activity (1990–1999), have been estimated to range from 6.8 to 19.1 per 100,000 per year, with an average of 13.8 [1]. This translates into an average of about 36,000 deaths per year. Estimates from other countries have varied according to the underlying cause of death chosen (all-causes, respiratory and circulatory diseases, or pneumonia and influenza causes of deaths). The estimate was similar in Hong Kong, where the average annual excess death rate associated with influenza was estimated at 12.4 per 100,000 based on cardio-respiratory conditions [2].
To the best of our knowledge, estimations of influenza-associated mortality have not been reported from arid and semi-arid countries, such as those in the Middle East. In addition, there is a need for more data on excess mortality rates in younger people, in view of the higher mortality rates observed in the younger age groups during the current A(H1N1) 2009 influenza pandemic [15]. The mortality data in Israel are essentially complete, and the sentinel clinics, together with sophisticated laboratory support, allow for reliable surveillance for influenza. Thus, there is a sound basis for estimating mortality associated with influenza. The purpose of the study reported here was to estimate the mortality attributed to seasonal influenza and to provide the basis for comparison with pandemic influenza.
Methods
Mortality data
Death notifications in Israel include demographic details, place and time of death, and immediate and contributory causes of death. The underlying cause of death is coded by the Central Bureau of Statistics (CBS). For this study, national monthly mortality data were obtained from the CBS. During the period under study, 1999–2006, the tenth revision of the International Classification of Diseases (ICD-10; National Center for Health Statistics, Hyattsville, MD) was used.
Laboratory data
In November 1996, a network of sentinel clinics was added to the influenza surveillance network, which has been existed since 1970’, by the Israel Center for Disease Control (ICDC) together with the National Influenza Center [16, 17]. Between October and April, swabs are routinely taken from a sample of cases with influenza-like illness (ILI) from the sentinel clinics and transported to the laboratory for virus isolation, typing, and sub-typing.
Statistical analysis
A respiratory year was defined as beginning on July 1 and ending on June 30 of the following year [1, 18]. For each year, the beginning of the period of influenza activity was defined by the month in which ≥10% [9] of the swabs were positive for influenza in 2 consecutive weeks. The end of the period was defined by the month in which <10% of the swabs were positive for influenza in 2 consecutive weeks or until the end of the surveillance period (in the case the positive sample rates were still >10%). Two weeks were added to the end of this period to include delayed deaths due to influenza. Since monthly mortality data were used, whole months were included, regardless of the number of weeks with influenza activity.
For each respiratory year in the study, we calculated total and age-specific excess mortality rates (number of deaths per 100,000 inhabitants) during the periods of influenza activity for three causes of death categories: all-causes, respiratory and circulatory (ICD-10 codes I00–I99, J00–J99) and deaths caused by influenza and pneumonia ICD-10 codes: J10–J18). The three age groups were: 0–49, 50–64, and ≥65 years. These three age groups were chosen due to a small number of deaths in the younger population and to adjust to the vaccination policy in Israel. The denominators used were the mean population sizes in each calendar year. For calculating rates for the respiratory year, we used an average of the two means of the populations that constructed each season.
Excess mortality rates during periods of influenza activity were calculated for each death category as follows:
- a ij :
-
= mortality rate during the jth month for the influenza period in the ith respiratory year
- b i :
-
= moving average mortality rate in the months with the lowest mortality rates in the ith respiratory year using 1 month prior to and 1 month following this month (if more than 1 minimum month was observed, the first was arbitrarily chosen)
- c j :
-
= average mortality rate difference (a ij –b i ) for the jth month in respiratory years with little influenza activity. Little influenza activity was defined as a season in which influenza isolations and ILI were below multi-year average
- E ij :
-
= excess mortality rate for the jth month in the ith respiratory year
The excess mortality rate for the ith respiratory year was then calculated as:
For each season, the 95% confidence intervals (95% CI) were calculated for the excess mortality using standard errors with normal approximation.
Results
The period of influenza activity in each respiratory year was found to be between December and April for seasons 1999–2002 and 2004–2005, and between October and February for 2003–2004. In most of the influenza seasons (1999–2000, 2001–2002, 2003–2004, 2004–2005), A(H3N2) was the dominant circulating subtype, except for season 2000–2001, during which A(H1N1) was the dominant circulating subtype. Since the influenza activity was extremely low in seasons 2002–2003 and 2005–2006, these years were chosen to represent excess winter mortality unrelated to influenza. In these seasons, the small amount of influenza reported was largely due to the influenza B subtype.
The estimated excess mortality rates and number of deaths according to cause of death associated with influenza activity for each respiratory year of the period studied and for all ages are shown in Table 1. The excess mortality rates from all-causes ranged between 7.7 (95% CI 5.9–9.4) and 36.1 (95% CI 30.4–41.8) per year, with an average of 21.2 (Table 1). This translated into 488–2,242 deaths, with an annual average of 1,386 deaths. The excess mortality rates per respiratory year from respiratory and circulatory diseases ranged from 4.4 (95% CI 3.8–5.0) and 24.4 (95% CI 21.5–27.4), with an average of 13.3. The excess number of deaths per respiratory year was between 280 and 1,516, with an annual average of 869 deaths. For pneumonia and influenza deaths, excess mortality rates were between −0.2 (95% CI −0.3 to −0.1) and 1.4 (95% CI 1.0–1.8), with an average of 0.6. The excess number of deaths per respiratory year ranged between −13 and 87, with an average of 37 deaths.
In most of the years, excess influenza-associated mortality rates were the highest among the elderly (age ≥65 years) (Table 2). The estimated excess rates from all-causes in the age group <50 years were between −0.08 and 4.3, with an average of 1.4. This translated into an average of 71 deaths (range −44 to 210). For the 50- to 64-year age group, excess rates from all-causes were between 9.3 and 34.2, with an average of 23.8 (translated into an average of 194 deaths, range 80–305), and among the oldest age group (≥65 years), these were between 45.3 and 292.8, with an average of 190.4 (translated into an average of 1,230 deaths, range 282–1,894).
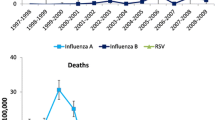
The average age-specific influenza associated mortality rates and number of deaths from respiratory and circulatory diseases by age group are presented in Fig. 1. Excess mortality rates from respiratory and circulatory diseases were: (1) in the 0- to 49-year age group, between −0.5 and 0.4, with an average of 0.14 (translated into an average of 7 deaths, range −25 and –19); (2) in the 50- to 64-year age group, between 1.9 and 13.9, with an average of 7 (translated into an average of 58 deaths, range 15–124); (3) in the oldest group, between 47.3 and 216.4, with an average of 119.9 (translated into an average of 772 deaths, range 295–1,316).
Most of the excess deaths from respiratory and circulatory diseases associated with influenza were observed among the elderly aged ≥65 years (average 89.9%, range 85.8–100%); in comparison, only 6.3% on average was observed among the 50- to 64-year age group. The excess number of deaths in the age group <50 years of age was negligible (average 1.35%, range −3.5 to 4.2%).
Of the total deaths (17,575), almost 5% (4.9%) on average (range 1.7–8.5%) was attributed to excess deaths from respiratory and circulatory diseases associated with influenza (all ages combined). In the age group <50 years, 6.9 excess deaths from respiratory and circulatory diseases were calculated, representing an average excess of 0.4% (range −1.6 to 1.1%) of the total deaths in this age group (1,683.2). In the age group 50–64 years, 58.1 excess deaths were calculated, representing an average excess of 2.97% (range 0.8–6%) of the total deaths (1,951.6). Among the elderly (≥65 years), the average excess was 5.5% (range 2.3–9.3%) (772.2 excess deaths and 13,940.6 total deaths in this age group).
Discussion
We found that, over the years surveyed, the estimated excess mortality rates during influenza epidemics varied by a factor of approximately 5 between respiratory years. However, the averages were remarkably similar to those reported for the USA [1]. Our estimates of 21.2 per 100,000 for all-causes, 13.3 for respiratory and circulatory diseases, and 0.6 for pneumonia and influenza are comparable to those of Thompson et al., who reported rates of 19.6, 13.8, and 3.1 respectively. The excess mortality varied markedly by age group, with most of the excess mortality observed in the elderly. For respiratory and circulatory diseases in the age group ≥65 years, the average for Israel was 119.9 compared with 98.3 per 100,000 in the USA, and in the 50- to 64-year age group, our average was 7 compared with 7.5 per 100,000 in the USA [1].
Since influenza can cause death directly through viral-induced acute respiratory distress syndrome (ARDS), complications from secondary infections, or by decompensation in patients with underlying chronic diseases [6], influenza-associated mortality is underestimated using the influenza and pneumonia diagnosis alone, as suggested by Thompson et al. [1]. It has been reported in Switzerland that the use of official influenza deaths underestimates the true influenza-related mortality by a factor of 2–3 among elderly aged ≥60 years [19]. On the other hand, the use of all-cause mortality may yield overestimates by including deaths not caused by influenza, as also suggested by Thompson et al. [1]. Thus, the use of deaths related to respiratory and circulatory diseases appears to provide the most reliable estimate [1]. The Centers for Disease Control and Prevention in the USA have adopted estimates for influenza-associated mortality based on excess deaths due to respiratory and circulatory causes [20].
Between-country comparisons of excess death rates based on all-cause mortality has some advantages since they are not dependent on death notification coding choices and physician behavior in completing the death notifications. Average annual excess death rates from all-causes during influenza epidemics in five countries have been reported to vary between 7.8 and 26 per 100,000 [1, 2, 10, 11, 21]. Some of these differences between countries may reflect differences in the extent of influenza morbidity as well as differences in vaccination coverage [22]. In most countries, the vaccine is given largely to the elderly and those with underlying chronic diseases, which may have attenuated the mortality rates. In addition, it has been found that during pandemic seasons, a high proportion of deaths occur among persons <65 years of age. However, during the immediately following epidemics seasons, deaths in this age group decline, suggesting long-term protection [23]. Thus, there appears to be immunological memory to similar viruses. Influenza-associated mortality rates appear to be related to the dominant virus subtype. Since its appearance in 1968, influenza A(H3N2) has been reported to be associated with increased mortality compared with influenza A(H1N1) or B [1, 8, 10, 24]. Other factors include the availability of supportive treatment, vaccine efficacy, and rates of chronic illnesses in the population.
The statistical methods used in the different studies carried out to date also vary [1, 2, 4, 8, 9, 25, 26]. However, although the methods may produce different estimates, in an assessment of four different models, similar estimates of influenza-associated deaths were obtained for those based on statistical models [9]. The method used for the estimates in our study is a variation of a method described by Thompson et al. [9] and is likely to give a more conservative estimate than the “peri-season” method described.
Limitations and strengths of the study
The study reported here has several limitations and strengths. Influenza virus circulates mainly in the winter months during which time temperatures are low; thus, some deaths attributed to influenza may in fact be related to the lower temperatures during the winter [12–14]. When computing the influenza-associated mortality rates, we deducted average excess mortality in two baseline years with little influenza activity in order to remove the effect of winter mortality unrelated to influenza. However, during those “baseline” years, there was also some influenza activity. Thus, we may have underestimated the excess influenza mortality. We used the underlying cause of death reported in the death notifications, as has been used by other researchers [1, 2, 24, 25]. The use of information on multiple causes of death could produce more accurate estimates, since the analysis would be less sensitive to coding choices for the underlying cause of death, as suggested by Dushoff et al. [18]. However, multiple causes of death data were not available.
The study has several strengths. The mortality data obtained were essentially complete, and the sentinel surveillance system by which the influenza period was defined is well-established. The influenza reference laboratory is certified by the World Health Organization (WHO).
Conclusions
These are the first systematic estimates of mortality attributed to seasonal influenza epidemics in Israel. While the estimates vary from year to year, the averages are strikingly similar to those obtained for the USA. In general, about 90% of influenza-associated deaths, from respiratory and circulatory diseases, occur in the age group 65+ years, and about 1% in the age group <50 years. This information underscores the major impact of the 2009 pandemic on the age group <50 years, and the importance of targeting the correct population for influenza vaccination programs. Deaths related to seasonal influenza were negligible among people <50 years, and although some countries recommend vaccinating this group, it should be emphasized that the vaccine will not have a significant impact on mortality; rather, it will have an effect on morbidity, preventing other age groups from being infected with the disease, and an economic impact (this group includes the working force of a community).
References
Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86.
Wong CM, Chan KP, Hedley AJ, Peiris JS. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39:1611–7.
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59.
Nicholson KG. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol Infect. 1996;116:51–63.
Neuzil KM, Reed GW, Mitchel EF Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–7.
Flahault A. First estimation of direct H1N1pdm virulence. From reported non consolidated data from Mauritius and New Caledonia. PLoS Curr Influenza: http://knol.google.com/k/antoine-flahault/first-estimation-of-direct-h1n1pdm/2nsp4xxomyqub/1#references. Accessed 27 Aug 2009.
Tsigrelis C, Mohammad M, Fraimow HS, Dellinger RP, Marchesani D, Reboli AC. Secondary bacterial pneumonia due to Staphylococcus aureus complicating 2009 influenza A (H1N1) viral infection. Infection. 2010;38:237–9.
Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50.
Thompson WW, Weintraub E, Dhankhar P, et al. Estimation of US influenza-associated deaths made using four different methods. Influenza Other Respi Viruses. 2009;3:37–49.
Rizzo C, Bella A, Viboud C, et al. Trends for influenza-related deaths during pandemic and epidemic seasons, Italy, 1969–2001. Emerg Infect Dis. 2007;13:694–9.
Zucs P, Buchholz U, Haas W, Uphoff H. Influenza associated excess mortality in Germany, 1985–2001. Emerg Themes Epidemiol. 2005;2:6.
Donaldson GC, Keatinge WR. Excess winter mortality: influenza or cold stress? Observational study. Br Med J. 2002;324:89–90.
Green MS, Harari G, Kristal-Boneh E. Excess winter mortality from ischaemic heart disease and stroke during colder and warmer years in Israel. Eur J Public Health. 1994;4:3–11.
Analitis A, Katsouyanni K, Biggeri A, et al. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol. 2008;168:1397–408.
World Health Organization. Preparing for the second wave: lessons from current outbreaks. 2009 Aug 28: http://www.who.int/csr/disease/swineflu/notes/h1n1_second_wave_20090828/en/index.html. Accessed 11 Oct 2009.
Shohat T, Versano N, Kiro A, Golan G, Mendelson E, Weingarten M. Use of sentinel reporting clinics for influenza surveillance in winter of 1996–1997 (in Hebrew). Harefuah. 1999;136:177–80.
Peled T, Weingarten M, Varsano N, et al. Influenza surveillance during winter 1997–1998 in Israel. Isr Med Assoc J. 2001;3:911–4.
Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States—an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–7.
Brinkhof MW, Spoerri A, Birrer A, Hagman R, Koch D, Zwahlen M. Influenza-attributable mortality among the elderly in Switzerland. Swiss Med Wkly. 2006;136:302–9.
Fiore AE, Sha DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines. Recommendation of the advisory committee on immunization practices (ACIP). MMWR 2009;58:RR-8: http://www.cdc.gov/mmwr/PDF/rr/rr5808.pdf. Accessed 11 Nov 2009.
Knycl J, Prochazka B, Goddard NL, et al. A study of excess mortality during influenza epidemics in the Czech Republic, 1982–2000. Eur J Epidemiol. 2005;20:365–71.
Blank PR, Schwenkglenks M, Szucs TD. Disparities in influenza vaccination coverage rates by target group in five European countries: trends over seven consecutive seasons. Infection 2009;37:390–400.
Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60.
Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–72.
Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160:492–502.
Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted at the Israel Center for Disease Control, Israel Ministry of Health, Tel Hashomer.
Rights and permissions
About this article
Cite this article
Linhart, Y., Shohat, T., Bromberg, M. et al. Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection 39, 399–404 (2011). https://doi.org/10.1007/s15010-011-0153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-011-0153-1