Abstract
Purpose
In 2007, a large goat-farming-associated Q fever outbreak occurred in the Netherlands. Data on the clinical outcome of Dutch Q fever patients are lacking. The current advocated follow-up strategy includes serological follow-up to detect evolution to chronic disease and cardiac screening at baseline to identify and prophylactically treat Q fever patients in case of valvulopathy. However, serological follow-up using commercially available tests is complicated by the lack of validated cut-off values. Furthermore, cardiac screening in the setting of a large outbreak has not been implemented previously. Therefore, we report here the clinical outcome, serological follow-up and cardiac screening data of the Q fever patients of the current ongoing outbreak.
Methods
The implementation of a protocol including clinical and serological follow-up at baseline and 3, 6 and 12 months after acute Q fever and screening echocardiography at baseline.
Results
Eighty-five patients with acute Q fever were identified (male 62%, female 38%). An aspecific, flu-like illness was the most common clinical presentation. Persistent symptoms after acute Q fever were reported by 59% of patients at 6 months and 30% at 12 months follow-up. We observed a typical serological response to Coxiella burnetii infection in both anti-phase I and anti-phase II IgG antibodies, with an increase in antibody titres up to 3 months and a subsequent decrease in the following 9 months. Screening echocardiography was available for 66 (78%) out of 85 Q fever patients. Cardiac valvulopathy was present in 39 (59%) patients. None of the 85 patients developed chronic Q fever.
Conclusions
Clinical, serological and echocardiographic data of the current ongoing Dutch Q fever outbreak cohort are presented. Screening echocardiography is no longer part of the standard work-up of Q fever patients in the Netherlands.
Similar content being viewed by others
Introduction
In late spring 2007, a large Q fever outbreak in the Netherlands occurred in the province of Noord Brabant, with a distinct epidemic centre around the town of Herpen [1]. Although no definitive source for the outbreak was identified, the preceding increased abortion rate amongst goats in the region suggested these ruminants as being the most likely reservoir [2, 3].
Q fever is a ubiquitous zoonosis caused by the obligate intracellular bacterium Coxiella burnetii. The clinical manifestations of this acute infection are usually self-limiting and range from a mild flu-like febrile illness to atypical pneumonia and hepatitis [4]. Serological and clinical follow-up after primary infection is recommended because approximately 1–5% of patients will develop chronic Q fever, endocarditis being the clinical manifestation in 60–70% of cases [5]. Cardiac valve abnormalities, vascular prosthesis, compromised immunity and pregnancy constitute predisposing host factors for chronic Q fever [6]. Recently, screening echocardiography has been added to the standard of care for Q fever patients [7, 8]. In daily clinical practice, however, adequate follow-up of patients after acute Q fever has practical difficulties. First, the interpretation of serology obtained by commercially available tests is hampered by incomplete knowledge of the natural course of the antibody response to Coxiella burnetii and the lack of validated cut-off values for chronic disease. Second, minor cardiac valvulopathies are frequently encountered in the general population, raising the question whether, indeed, all patients with cardiac valve abnormalities should receive prolonged prophylactic antibiotic treatment.
Faced with the aforementioned Q fever outbreak in the Netherlands, a follow-up protocol was implemented, including clinical and serological follow-up for a 1-year period, and Q fever patients were offered a screening echocardiography at baseline. The aim of this paper was to report the clinical characteristics and outcome, serological data and echocardiographic findings of the current ongoing Q fever outbreak in the Netherlands.
Methods
Q fever case definition
A case of acute Q fever was defined as any inhabitant of the outbreak cluster area who presented with one or more compatible clinical symptoms (fever, fatigue, chills, headache, myalgia, sweats, cough [4]) and the demonstration of infection with Coxiella burnetii, as evidenced by: (1) a seroconversion or four-fold increase of antibody titre using a Coxiella burnetii complement fixation test (CFT) in samples taken at least 14 days apart, (2) the presence of both anti-phase II IgM and anti-phase II IgG antibodies in the Coxiella burnetii immunofluorescence assay (IFA) with a 1:64 or greater dilution [1] or a positive serum polymerase chain reaction (PCR). For patients admitted to hospital and presenting with pneumonia, the severity of disease was assessed using the pneumonia severity index (PSI) [9].
A case of chronic Q fever is defined as any inhabitant of the outbreak cluster area with a clinical entity compatible with chronic Coxiella burnetii infection as described in the literature by Raoult (endocarditis, vascular infection, osteoarticular infection, chronic hepatitis, pregnancy), in the presence of an anti-phase I IgG titre ≥800, for ≥6 months after the initial day of illness [4, 10].
Follow-up protocol
The follow-up protocol consisted of a complete history and physical examination at 6 and 12 months after the initial day of illness, serological testing at baseline, followed by testing after 3, 6 and 12 months after a referral to a cardiologist for a single screening transthoracic echocardiogram. Data on symptoms were obtained by asking the patient an open question on the presence of any complaints. No structured questionnaire was used. As the Q fever outbreak was identified retrospectively, data on presenting symptoms at baseline were collected through the review of all available medical records at the GP practice. Since this concerned an observational study, all interventions had been part of the standard care. Therefore, patients were asked to co-operate and no specific ethical approval for this study was sought.
Serology and polymerase chain reaction
Sera were tested for Coxiella burnetii antibodies using a CFT (Institute Virion/Serion, GmbH, Würzburg, Germany), testing only anti-phase II antibodies, and an IFA (Focus Diagnostics, Cypress, CA, USA), assessing IgM and IgG antibodies to both phase I and II antibodies. Sera taken at baseline (T = 0) were also tested by PCR as described in the literature [11]. The respective time-points for final analysis were defined as follows: baseline (T = 0) is the date of the first available serological results within 6 weeks after the first day of illness; serological results at 3 (T = 3), 6 (T = 6) and 12 months (T = 12) after the first day of illness were included if blood samples were drawn at these time-points, with a margin of plus or minus 1 month.
Screening echocardiography
Structural cardiac abnormalities and valvular defects were classified according to the American Society of Echocardiography (ASE) guidelines [12–15]. The ASE guidelines are a consensus statement of the American College of Cardiology, the American Heart Association and the European Society of Cardiology. These guidelines provide a framework for the standardised assessment of the severity of valvular regurgitation and stenosis, using well-defined structural, Doppler and quantitative echocardiographic parameters. Major (or clinically significant) valvulopathies are defined as moderate and severe regurgitation or stenosis of the mitral and/or aortic valve. Minor valvulopathies are defined as trace or mild regurgitation or stenosis of the mitral and/or aortic valve, a bicuspid aortic valve and mitral valve prolapse without significant accompanying stenosis or regurgitation. Data to calculate percentages were analysed using Microsoft Excel 7.0 and SPSS 17.0. GraphPad Prism 4.0 was used to generate Fig. 1.
Results
The Herpen Q fever outbreak cohort
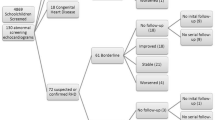
A total of 85 patients with acute Q fever were identified in the outbreak cluster. The patient characteristics are given in Table 1. The male-to-female ratio was 1.7. None of the female patients was pregnant. Co-morbidity was present in 26 patients (31%). Six patients had a known risk factor for developing chronic Q fever: four patients with previously documented significant cardiac valvulopathies, one patient using long-term high-dose corticosteroids for idiopathic thrombocytopenic purpura and one patient with an aortic vascular prosthesis. Complete baseline and follow-up data on symptoms and physical examinations were available for all patients and are given in Table 2.
An aspecific, flu-like illness was the most common clinical presentation. All patients who were admitted to hospital (8 female, 16 male) presented with (atypical) pneumonia and 83% of these patients had a PSI of class I or class II, representing a low disease severity [9]. At 6 months follow-up, more than half of all Q fever patients had persistent symptoms, fatigue being the most prevalent complaint. At one year, reports of persistent symptoms had roughly halved. Twenty-six percent of patients still reporting fatigue which they attributed to their acute Q fever episode. None of the 85 patients had developed chronic Q fever at the 1-year follow-up point. One male patient died of a myocardial infarction at 8 months follow-up, unrelated to Q fever.
Serology
Serological data were available at baseline (68%), at 3 months follow-up (49%), at 6 months follow-up (81%) and at 12 months follow-up (75%). The results are shown in Fig. 1. CFT and IFA showed similar profiles, but at a different titre level. The numbers of patients with an anti-phase I IgG titre equal to or more than 800 (suggesting chronic disease) at the respective time-points were 7 (T = 0), 21 (T = 3), 13 (T = 6) and 2 (T = 12). PCR was performed on 60 out of 85 first available sera. Seven of these 60 patients (11.6%) had a positive PCR and negative serology. All PCR-positive patients subsequently seroconverted after 3 months.
Echocardiography
Screening transthoracic echocardiography was available for 66 (78%) out of 85 Q fever patients. The remaining 19 patients repeatedly failed to show up at their appointment for a transthoracic echocardiogram. The results are shown in Table 3.
Cardiac valvulopathy was present in 39 (59%) patients, five of whom had a major or clinically significant (moderate or severe) valvulopathy. Four of these five patients were previously known to have a cardiac valvulopathy. One patient had a bicuspid aortic valve associated with a moderate aortic insufficiency. This anomaly was classified as a clinically significant valvulopathy. The remaining 34 patients had one or more minor cardiac valvular abnormalities.
Discussion
As expected, an aspecific, flu-like syndrome with or without signs of respiratory tract infection was the main clinical Q fever manifestation in this remarkably compliant cohort of patients. However, chest radiography was only performed in patients admitted to hospital, which could underestimate the prevalence of pneumonia in patients treated by their GP. Males were more likely to suffer from symptomatic Q fever than females, which is a well-known phenomenon and appears to be the result of the gender-related expression of sex hormones [16].
Fatigue was noted by Q fever patients in 52% at 6 months and 26% at 1 year following primary infection. Other research groups, using various questionnaires, have reported a comparably high proportion of fatigued patients [17–21]. However, fatigue levels in this outbreak cohort are self-reported and a control group is lacking. Therefore, these findings must be interpreted with extreme caution. Further studies are needed to evaluate the long-term symptoms and quality of life in Q fever patients in the Netherlands.
Using a commercially available IFA, we observed a typical serological response to Coxiella burnetii infection with strikingly high levels of antibodies to both phase I and phase II antibodies. This serological response was characterised by an increase in antibody titres up to 3 months and a subsequent decrease in the following 9 months. The anti-phase I IgG kinetic, with a titre peak at 3 months, is different from the available literature on IFA serological patterns in the follow-up of acute Q fever, which shows a rather slow and gradual increase of anti-phase I IgG antibodies [22, 23]. The high proportion of Q fever patients in this cohort that received a beta-lactam antibiotic with no efficacy against Coxiella burnetii (38%) or no treatment at all (14%) could possibly account for this discrepancy. The CFT and the IFA tests showed an identical serological pattern over time. Therefore, both tests might be useful for the follow-up of patients. Additional IFA testing should be performed when the CFT titre is rising in order to observe the balance between anti-phase I and anti-phase II IgG antibodies.
Although at the various time-points there were patients with an anti-phase I IgG antibody titre of 1,024 or more, suggesting chronic disease, none of these patients developed a clinical picture compatible with chronic Q fever. Furthermore, at follow-up, all of these patients showed a spontaneous subsequent decline in anti-phase I IgG titres, which, in the case of a possible chronic Q fever, is highly unlikely, given the natural detrimental course of chronic disease if left untreated.
For aiding in the diagnosis of chronic Q fever, we used the well-established anti-phase I IgG antibody titre of ≥800 [10]. Unfortunately, this cut-off value is based on a single-centre experience using a home-made IFA test. Additional studies are urgently needed to compare both tests and to give cut-off levels that can be used with commercially available IFA tests.
Q fever endocarditis may develop in up to 39% of patients with known pre-existing valvulopathy [24]. Even minor cardiac valve abnormalities might be a risk factor for Q fever endocarditis [7]. An active search for cardiac valvular abnormalities and serological surveillance following acute Q fever have been advocated to identify chronic Q fever in an early stage and to trigger prolonged prophylactic antibiotic treatment in case of even minor cardiac valvulopathy [7, 8, 25].
No cases of chronic Q fever were observed during the follow-up, despite the high incidence of minor valvulopathies found by screening echocardiography. Similar incidences of minor cardiac valvular abnormalities in the general population have been reported in large series [26, 27]. For example, the prevalence of ‘trace’ mitral valve regurgitation is 40% and can, therefore, be considered to be a physiological phenomenon without clinical importance [12]. The prevalence of mitral valve prolapse and bicuspid aortic valve is estimated to be 2–3% and circa 1%, respectively [28, 29]. The absolute risk of developing chronic Q fever for these minor valvulopathies remains unknown. The limited number of patients in this study does not allow to determine this risk, but it is clear that the absolute risk, at least in the case of a minor mitral valve insufficiency, is likely to be small enough to withhold prolonged prophylactic antibiotic treatment and closely monitor serology during the follow-up.
In the setting of the ongoing Q fever epidemic in the Netherlands that currently encompasses more than 3,000 new Q fever patients, performing screening echocardiograms in all patients is costly [30]. Moreover, it is not likely that such a screening will detect cardiac valve abnormalities that, in turn, would influence clinical management. Therefore, we currently perform only serological and clinical follow-up after acute Q fever, omitting routine screening echocardiography. In other words, in the context of the large and continuous outbreak in the Netherlands, a pragmatic approach has been adopted for patients without known risk factors for chronic disease, consisting of close serological monitoring for a period of 1 year (at 3, 6 and 12 months). Only when serological and/or clinical signs of chronic disease appear is further investigation using PCR and echocardiography undertaken. In patients with known, pre-existing risk factors for chronic disease, including cardiac valvulopathy, decisions regarding follow-up and prophylactic antibiotic treatment are made in each individual case by a multi-disciplinary team including a medical microbiologist, infectiologist and cardiologist. We realise that such a strategy can only be applied in countries with a low background prevalence of cardiac valvulopathies in the general population. Indeed, in India, due to the high prevalence of rheumatic heart disease, Coxiella burnetii is responsible for 14% of culture-negative endocarditis cases [31].
In conclusion, in the Dutch Q fever outbreak, an aspecific febrile illness with or without respiratory tract symptoms was the most common clinical presentation. Fatigue was present in 52% of patients at 6 months and this dropped to 26% at 1-year follow-up. Using a commercially available IFA, we observed a typical serological response to both phase I and phase II Coxiella burnetii antigens, characterised by an increase in antibody titres up to 3 months and a subsequent decrease in the following 9 months. Screening echocardiography at baseline revealed cardiac valve abnormalities in 59% of patients investigated, with only 7.6% having a clinically significant valvulopathy. None of the patients progressed to chronic disease. Baseline screening echocardiography is no longer part of the standard work-up of Q fever patients the Netherlands.
References
Karagiannis I, Morroy G, Rietveld A, et al. Q fever outbreak in the Netherlands: a preliminary report. Euro Surveill. 2007;12:E070809.2.
Van Steenbergen JE, Morroy G, Groot CA, et al. An outbreak of Q fever in The Netherlands—possible link to goats. Ned Tijdschr Geneeskd. 2007;151:1998–2003.
Karagiannis I, Schimmer B, Van Lier A, et al. Investigation of a Q fever outbreak in a rural area of The Netherlands. Epidemiol Infect. 2009;137:1283–94.
Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–53.
Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006;367:679–88.
Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–26.
Fenollar F, Thuny F, Xeridat B, et al. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clin Infect Dis. 2006;42:818–21.
Landais C, Fenollar F, Thuny F, et al. From acute Q fever to endocarditis: serological follow-up strategy. Clin Infect Dis. 2007;44:1337–40.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50.
Fournier PE, Casalta JP, Habib G, et al. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–33.
Fournier PE, Raoult D. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J Clin Microbiol. 2003;41:5094–8.
Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802.
Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
Leone M, Honstettre A, Lepidi H, et al. Effect of sex on Coxiella burnetii infection: protective role of 17beta-estradiol. J Infect Dis. 2004;189:339–45.
Marmion BP, Shannon M, Maddocks I, et al. Protracted debility and fatigue after acute Q fever. Lancet. 1996;347:977–8.
Ayres JG, Smith EG, Flint N. Protracted fatigue and debility after acute Q fever. Lancet. 1996;347:978–9.
Wildman MJ, Smith EG, Groves J, et al. Chronic fatigue following infection by Coxiella burnetii (Q fever): ten-year follow-up of the 1989 UK outbreak cohort. QJM. 2002;95:527–38.
Hatchette TF, Hayes M, Merry H, et al. The effect of C. burnetii infection on the quality of life of patients following an outbreak of Q fever. Epidemiol Infect. 2003;130:491–5.
Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
Dupuis G, Peter O, Peacock M, et al. Immunoglobulin responses in acute Q fever. J Clin Microbiol. 1985;22:484–7.
Dupont HT, Thirion X, Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–96.
Fenollar F, Fournier PE, Carrieri MP, et al. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6.
Million M, Lepidi H, Raoult D. Q fever: current diagnosis and treatment options. Med Mal Infect. 2009;39:82–94.
Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897–902.
Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol. 2001;87:298–304.
Lewin MB, Otto CM. The bicuspid aortic valve: adverse outcomes from infancy to old age. Circulation. 2005;111(7):832–4.
Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–18.
Schimmer B, Dijkstra F, Vellema P, et al. Sustained intensive transmission of Q fever in the south of the Netherlands, 2009. Euro Surveill. 2009;14:19210.
Balakrishnan N, Menon T, Fournier PE, et al. Bartonella quintana and Coxiella burnetii as causes of endocarditis, India. Emerg Infect Dis. 2008;14:1168–9.
Acknowledgments
The authors are greatly indebted to the GPs who helped to follow up the outbreak cohort, the personnel at the Herpen GPs office “Huisartsenpraktijk Herpen” for their assistance and the laboratory personnel at the Jeroen Bosch Hospital and the Canisius-Wilhelmina Hospital for aiding in the serological testing. We thank all of the personnel at the Municipal Health Service “Hart voor Brabant” for their contribution to the clinical follow-up. We thank Dr. Zwart, cardiologist at Bernhoven Hospital location Oss and the personnel of the cardiac function test laboratory at Bernhoven Hospital for providing the echocardiographic data and Prof. em. Ben de Pauw for the critical review of the manuscript.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Limonard, G.J.M., Nabuurs-Franssen, M.H., Weers-Pothoff, G. et al. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: clinical, serological and echocardiographic findings. Infection 38, 471–477 (2010). https://doi.org/10.1007/s15010-010-0052-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-010-0052-x