Abstract
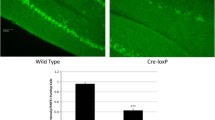
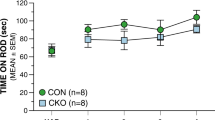
Previously, deficiency in the expression of the nuclear orphan receptor TAK1 was found to be associated with delayed cerebellar granule cell migration and Purkinje cell maturation with a permanent deficit in foliation of lobules VI–VII, suggesting a role for TAK1 in cerebellum development. In this study, we confirm that TAK1-deficient (TAK1−/−) mice have a smaller cerebellum and exhibit a disruption of lobules VI–VII. We extended these studies and show that at postnatal day 7, TAK1−/− mice exhibit a delay in monolayer maturation of dysmorphic calbindin 28K-positive Purkinje cells. The astrocyte-specific glutamate transporter (GLAST) was expressed within Bergmann fibers and internal granule cell layer at significantly lower levels in the cerebellum of TAK1−/− mice. At PND21, Golgi-positive Purkinje cells in TAK1−/− mice displayed a smaller soma (18%) and shorter distance to first branch point (35%). Neuronal death was not observed in TAK1−/− mice at PND21; however, activated microglia were present in the cerebellum, suggestive of earlier cell death. These structural deficits in the cerebellum were not sufficient to alter motor strength, coordination, or activity levels; however, deficits in acoustic startle response, prepulse startle inhibition, and social interactions were observed. Reactions to a novel environment were inhibited in a light/dark chamber, open-field, and home-cage running wheel. TAK1−/− mice displayed a plateau in performance on the running wheel, suggesting a deficit in learning to coordinate performance on a motor task. These data indicate that TAK1 is an important transcriptional modulator of cerebellar development and neurodevelopmentally regulated behavior.











Similar content being viewed by others
Abbreviations
- BrdU:
-
Bromodeoxyuridine
- CAPS2:
-
Ca2+-dependent activator protein for secretion 2
- DAB:
-
3,3′-Diaminobenzidine
- DNER:
-
Delta/notch-like epidermal growth factor repeat
- EGL:
-
External granular layer
- GLAST:
-
L-glutamate/L-aspartate transporter
- IGL:
-
Internal granular layer
- MZ:
-
Marginal zone
- ML:
-
Molecular layer
- NMDA:
-
N-methyl-d-aspartic acid
- PND:
-
Postnatal day
References
Chang C, Da Silva SL, Ideta R, Lee Y, Yeh S, Burbach JP (1994) Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc Natl Acad Sci U S A 91:6040–6044
Hirose T, Fujimoto W, Tamaai T, Kim KH, Matsuura H, Jetten AM (1994) TAK1: molecular cloning and characterization of a new member of the nuclear receptor superfamily. Mol Endocrinol 8:1667–1680
Yoshikawa T, DuPont BR, Leach RJ, Detera-Wadleigh SD (1996) New variants of the human and rat nuclear hormone receptor, TR4: expression and chromosomal localization of the human gene. Genomics 35:361–366
Hirose T, O'Brien DA, Jetten AM (1995) Cloning of the gene encoding the murine orphan receptor TAK1 and cell-type-specific expression in testis. Gene 163:239–242
Lee YF, Pan HJ, Burbach JP, Morkin E, Chang C (1997) Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes. J Biol Chem 272:12215–12220
Yan ZH, Karam WG, Staudinger JL, Medvedev A, Ghanayem BI, Jetten AM (1998) Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4. J Biol Chem 273:10948–10957
Hirose T, Apfel R, Pfahl M, Jetten AM (1995) The orphan receptor TAK1 acts as a repressor of RAR-, RXR- and T3R-mediated signaling pathways. Biochem Biophys Res Commun 211:83–91
Yang Y, Wang X, Dong T, Kim E, Lin WJ, Chang C (2003) Identification of a novel testicular orphan receptor-4 (TR4)-associated protein as repressor for the selective suppression of TR4-mediated transactivation. J Biol Chem 278:7709–7717
Nakajima T, Fujino S, Nakanishi G, Kim YS, Jetten AM (2004) TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res 32:4194–4204
van Schaick HS, Rosmalen JG, Lopes da Silva S, Chang C, Burbach JP (2000) Expression of the orphan receptor TR4 during brain development of the rat. Brain Res Mol Brain Res 77:104–110
Chen YT, Collins LL, Chang SS, Chang C (2008) The roles of testicular orphan nuclear receptor 4 (TR4) in cerebellar development. Cerebellum 7:9–17
Hatten ME (2002) New directions in neuronal migration. Science 297:1660–1663
Rakic P, Sidman RL (1973) Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A 70:240–244
Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y (1997) Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci 17:9212–9219
Goldowitz D (1989) The weaver granuloprival phenotype is due to intrinsic action of the mutant locus in granule cells: evidence from homozygous weaver chimeras. Neuron 2:1565–1575
Hamre KM, Goldowitz D (1997) Meander tail acts intrinsic to granule cell precursors to disrupt cerebellar development: analysis of meander tail chimeric mice. Development 124:4201–4212
Ross ME, Fletcher C, Mason CA, Hatten ME, Heintz N (1990) Meander tail reveals a discrete developmental unit in the mouse cerebellum. Proc Natl Acad Sci U S A 87:4189–4192
Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M (2000) Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol 418:106–120
Dahmane N, Ruiz i Altaba A (1999) Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126:3089–3100
Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP (2004) Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol 270:393–410
Aruga J, Inoue T, Hoshino J, Mikoshiba K (2002) Zic2 controls cerebellar development in cooperation with Zic1. J Neurosci 22:218–225
Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M (2005) DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci 8:873–880
Tohgo A, Eiraku M, Miyazaki T, Miura E, Kawaguchi SY, Nishi M, Watanabe M, Hirano T, Kengaku M, Takeshima H (2006) Impaired cerebellar functions in mutant mice lacking DNER. Mol Cell Neurosci 31:326–333
Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T (2007) Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci 27:2472–2482
Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C (2004) Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci U S A 101:15058–15063
Harry GJ, Funk JA, Lefebvre d'Hellencourt C, McPherson CA, Aoyama M (2008) The type 1 interleukin 1 receptor is not required for the death of murine hippocampal dentate granule cells and microglia activation. Brain Res 1194:8–20
Harry GJ, Kraft AD (2008) Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol 4:1265–1277
Fox MW (1965) Environmental factors influencing stereotyped and allelomimetic behavior in animals. Lab Anim Care 15:363–370
Fox WM (1965) Reflex-ontogeny and behavioural development of the mouse. Anim Behav 13:234–241
Harry GJ, Tilson HA (1981) The effects of postpartum exposure to triethyl tin on the neurobehavioral functioning of rats. Neurotoxicology 2:283–296
Dulawa SC, Geyer MA (2000) Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology 39:2170–2179
Geyer MA, McIlwain KL, Paylor R (2002) Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry 7:1039–1053
Moy SS, Nadler JJ, Magnuson TR, Crawley JN (2006) Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genet C Semin Med Genet 142C:40–51
Marler MR, Gehrman P, Martin JL, Ancoli-Israel S (2006) The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med 25:3893–3904
Chen YT, Collins LL, Uno H, Chou SM, Meshul CK, Chang SS, Chang C (2007) Abnormal cerebellar cytoarchitecture and impaired inhibitory signaling in adult mice lacking TR4 orphan nuclear receptor. Brain Res 1168:72–82
Alcantara S, Ferrer I, Soriano E (1993) Postnatal development of parvalbumin and calbindin D28K immunoreactivities in the cerebral cortex of the rat. Anat Embryol (Berl) 188:63–73
Iacopino AM, Rhoten WB, Christakos S (1990) Calcium binding protein (calbindin-D28k) gene expression in the developing and aging mouse cerebellum. Brain Res Mol Brain Res 8:283–290
Chen YT, Collins LL, Uno H, Chang C (2005) Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol Cell Biol 25:2722–2732
Zhang Y, Chen YT, Xie S, Wang L, Lee YF, Chang SS, Chang C (2007) Loss of testicular orphan receptor 4 impairs normal myelination in mouse forebrain. Mol Endocrinol 21:908–920
Willuhn I, Steiner H (2006) Motor-skill learning-associated gene regulation in the striatum: effects of cocaine. Neuropsychopharmacology 31:2669–2682
Cook EH Jr, Courchesne RY, Cox NJ, Lord C, Gonen D, Guter SJ, Lincoln A, Nix K, Haas R, Leventhal BL, Courchesne E (1998) Linkage-disequilibrium mapping of autistic disorder, with 15q11–13 markers. Am J Hum Genet 62:1077–1083
Perry W, Minassian A, Lopez B, Maron L, Lincoln A (2007) Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61:482–486
McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG (2002) Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain 125:1594–1606
Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T (2002) Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol 14:581–611
Moy SS, Nadler JJ (2008) Advances in behavioral genetics: mouse models of autism. Mol Psychiatry 13:4–26
Swerdlow NR, Shoemaker JM, Stephany N, Wasserman L, Ro HJ, Geyer MA (2002) Prestimulus effects on startle magnitude: sensory or motor? Behav Neurosci 116:672–681
Paylor R, Crawley JN (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 132:169–180
Boyden ES, Katoh A, Raymond JL (2004) Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609
Ito M (2008) Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–313
Rio C, Rieff HI, Qi P, Khurana TS, Corfas G (1997) Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 19:39–50
Zheng C, Heintz N, Hatten ME (1996) CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science 272:417–419
Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME (2002) Mice that lack astrotactin have slowed neuronal migration. Development 129:965–972
Nicholls D, Attwell D (1990) The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11:462–468
Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K (1998) Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci 10:976–988
De Schutter E, Vos B, Maex R (2000) The function of cerebellar Golgi cells revisited. Prog Brain Res 124:81–93
Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y (2002) Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-d-aspartate receptor activity in hippocampal CA3 circuits. J Cell Biol 158:215–220
Goldowitz D, Hamre K (1998) The cells and molecules that make a cerebellum. Trends Neurosci 21:375–382
Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501
Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, Olivier B (2002) Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry 51:583–590
Fiez JA (1996) Cerebellar contributions to cognition. Neuron 16:13–15
Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT (1996) Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 272:545–547
Acknowledgements
The authors thank Dr. Randy Thresher, UNC/Chapel Hill, NC for generating TAK1−/− mice; Ms. Laura M. Degraff for technical assistance; Dr. Fu Du for the Golgi staining, and Drs. Gordon Flake (NIEHS) and Robert Berman (University of California/Davis) for comments. This research was supported by the Division of Intramural Research at the National Institute of Environmental Health Sciences, NIH (Z01-ES-101586; Z01-ES-021164).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, YS., Harry, G.J., Kang, H.S. et al. Altered Cerebellar Development in Nuclear Receptor TAK1/TR4 Null Mice Is Associated with Deficits in GLAST+ Glia, Alterations in Social Behavior, Motor Learning, Startle Reactivity, and Microglia. Cerebellum 9, 310–323 (2010). https://doi.org/10.1007/s12311-010-0163-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-010-0163-z