Abstract
The myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS) occasionally demonstrate overlapping morphological features including hypercellularity, mild/nonspecific dysplastic changes and variable bone marrow fibrosis. Thus, when the associated bone marrow fibrosis results in a suboptimal specimen for morphological evaluation, the descriptive diagnosis “fibrotic marrow with features indeterminate for MDS versus MPN” is often applied. The JAK2 V617F mutation was recently shown to be frequently identified in MPN, but it is rarely present in other myeloid disorders. However, the diagnostic utility of JAK2 V617F screening in hypercellular bone marrow specimens with fibrosis has not been previously investigated. Using a real-time polymerase chain reaction melting-curve assay capable of detecting JAK2 V617F in archived fixed materials, we retrospectively studied JAK2 V617F in 45 cases with fibrotic hypercellular bone marrow at initial presentation, including 19 cases initially described as “with features indeterminate for MDS versus MPN”. These 19 cases were reclassified into more specific categories of MDS (n = 14) or MPN (n = 5) based on the availability of subsequent clinical data and/or bone marrow examinations. The JAK2 V617F allele was identified in 17 out of 18 BCR/ABL gene-negative MPN cases with marrow fibrosis, whereas only wild-type alleles were identified in the remaining non-MPN cases. Importantly, JAK2 V617F alleles were seen in all five cases of “with features indeterminate for MDS versus MPN” at initial presentation that were later determined to be MPN, but they were absent in the 14 cases later determined to be MDS. Our results suggest that JAK2 V617F allele evaluation can be a useful ancillary test for discriminating MDS from MPN in specimens with bone marrow fibrosis.
Similar content being viewed by others
Introduction
The myeloproliferative neoplasms (MPN, also known as chronic myeloproliferative disorders or MPD) and myelodysplastic syndromes (MDS) are usually distinguished by their clinical presentation, laboratory parameters, and morphological appearance. However, they occasionally demonstrate overlapping features including the coexisting presence of mild hypercellularity, mild/nonspecific dysplasia, and variable bone marrow fibrosis [1, 2]. Cases with fibrosis may be problematic due to the difficulties associated with obtaining an adequate aspirate smear specimen for optimal microscopic examination, particularly when complete clinical information and/or a peripheral blood smear is not available. In these cases, descriptive diagnoses, such as “with features indeterminate for MDS versus MPN”, are usually given, and follow-up biopsies may be necessary for rendering specific diagnoses. New molecular markers that better discriminate these morphologically similar but biologically distinct entities could significantly improve clinical management and facilitate research studies by providing accurate diagnoses at the time of initial presentation [1–3].
A specific mutation in the Janus kinase 2 gene (JAK2 V617F) was recently shown to be frequently and preferentially identified in the bone marrow and peripheral blood cells of MPN patients [1, 2, 4–20]. The JAK2 V617F allele has been detected in the vast majority of polycythemia vera (PV) cases, in the majority of essential thrombocytosis (ET) and primary myelofibrosis (PMF) cases, and in many acute leukemias representing transformation from preexisting MPN. However, JAK2 V617F is rarely identified in healthy controls or patients with other myeloid disorders. Thus, JAK2 V617F has general diagnostic value for MPN, but it cannot be used to differentiate between PV, ET, or PMF [1, 2, 4–19].
The diagnostic utility of JAK2V617F mutation screening in hypercellular bone marrow specimens with fibrosis has not been previously investigated. We retrospectively evaluated the JAK2 genotype of 45 fibrotic bone marrow specimens, including 19 cases that were originally diagnosed as “with features indeterminate for MDS versus MPN” using our assay that reliably detects JAK2 V617F in archived and paraffin-embedded materials [20, 21]. Our results demonstrated that the presence or absence of JAK2V617F may have diagnostic implications for these cases.
Materials and methods
Patient samples
Archival pathology and hematology records at our respective institutions were retrospectively reviewed to identify patients with a mildly to markedly fibrotic bone marrow biopsy at initial marrow evaluation. Using reticulin and collagen stains, fibrosis was graded on a scale of 0 to 3 as previously described [22]. For PMF cases, grade 1 was considered the early/prefibrotic stage (also termed cellular phase) and grades 2–3 was considered the fibrotic stage [22, 23]. The cohort was limited to nonchronic myelogenous leukemia (BCR/ABL fusion gene negative) patients with adequate history and follow-up for clinicopathologic correlation. Forty-five specimens were identified as follows: 19 cases initially assigned to “with features indeterminate for MDS versus MPN” because of fibrosis-associated inadequate aspirate and/or lack of complete clinical information, 11 cases with a confirmed MPN (two PMF prefibrotic stage, four PMF fibrotic stage, three PV at spent phase, two ET, and four PMF), two cases with acute myelogenous leukemia (AML) transformed from a preexisting PMF, two cases with chronic myelomonocytic leukemia, and 11 cases with other neoplastic myeloid disorders (Table 1). None of these cases were tested for JAK2 mutation at the time of initial evaluation. This study was approved by the Institutional Review Board of all participating institutions, and samples were obtained in accordance with institutional policies.
DNA extraction and real-time PCR melting curve analysis for JAK2 genotype
For each case, DNA was extracted from stained or unstained peripheral blood smears, stained or unstained bone marrow aspirate smears, or formalin-fixed paraffin-embedded bone marrow clot sections using the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA) as previously described [20, 21]. There was a relatively even distribution of each specimen type within each disease category (Table 1). Real-time polymerase chain reaction (PCR) melting curve analysis for the JAK2 wild-type and V617F mutant allele was performed on a LightCycler platform (Roche Applied Diagnostics, Indianapolis, IN, USA) using primers and probes as previously described [20, 21]. Briefly, PCR primers were designed to flank codon 617 of the JAK2 gene, including forward primer JAKLCFP 5′-AAg CAg CAA gTA TgA TgA gCA A-3′ and reverse primer JAKLCRP 5′-AgC TgT gAT CCT gAA ACT gAA-3′. FRET probes were designed with the 5′ probe overlapping the mutated codon and the 3′ probe annealing immediately downstream, including LCRD 5′-640-CAg ACA CAT ACT CCA TAA TTT-3′ and LCFN 5′-gTA gTT TTA CTT ACT CTC gTC TC-FITC-3′. Real-time PCR was performed on each specimen using 5.0 μL of purified DNA extract in a total reaction volume of 20 μL that included 4 μL of FastStart DNA MasterPLUS SYBR Green I 5× reaction master mix (Roche Applied Science), 2.0 μL JAK2LCFP (final concentration 0.5 μM), 2.0 μL JAK2LCRP (final concentration 0.5 μM), 1.0 μL LCFN (final concentration 0.5 μM), 1.0 μL LCRD (final concentration 0.5 μM), and 5.0 μL nuclease-free water. The PCR cycle parameters were one initial denaturing step of 95°C for 10 min and 55 cycles consisting of 95°C for 10 s, 60°C for 60 s, and 75°C for 10 s. The DNA melting curve analysis was performed by denaturing at 95°C for 10 s, annealing at 29°C for 60 s, and melting by a transition rate of 0.20 C/s to 70 C. Melting curves were visually analyzed, and the melting temperature (T m) of each sample was electronically recorded. Homozygous mutant (JAK2 V617F/JAK2 V617F) human erythroleukemia (HEL) and homozygous wild-type (JAK2/JAK2) multiple myeloma (RPMI8226) cell lines were used as positive and negative controls, respectively (Fig. 1).
Representative JAK2 real-time PCR melting curves. Melting curves are drawn with −dF/dT on the y-axis and melting temperature (T M,°C) on the x-axis. The homozygous wild-type (JAK2/JAK2) multiple myeloma control cell line (RPMI 8226) is shown in red with the melting curve peak at approximately 56°C (T M = 56°C), and the homozygous mutant (JAK2 V617F/JAK2 V617F) human erythroleukemia control cell line (HEL) is shown in blue with the melting curve peak at approximately 47°C (T M = 47°C). A representative case of MDS-F (JAK2 wild type) is shown in yellow with a single T M equivalent to the wild-type JAK2 allele. A representative case of prefibrotic PMF (heterozygous JAK2/JAK2 V617F) is shown in green with two T M; one peak corresponds to the JAK2 wild-type allele and the other corresponds to the JAK2 V617F mutant allele
Results
Medical records and bone marrow specimens obtained at subsequent evaluations were reviewed for all the patients to render final diagnoses using World Health Organization criteria to the fullest extent possible [1, 23–25]. Of note, the 19 cases initially designated as “with features indeterminate for MDS versus MPN” were ultimately reclassified as either PMF (n = 5) or MDS with myelofibrosis (MDS-F, n = 14; Table 1) based on repeat/follow-up biopsies and/or clinical progression. None of these cases fulfilled the criteria for the diagnosis of MDS/MPN according to WHO classification. The blast count in the cases finally diagnosed as MDS-F could not be accurately evaluated due to inadequate marrow smear, and thus, a definite subtype of MDS could not be assigned accurately. However, the CD34 stain showed an increase of blasts in the majority of these cases, suggesting they were high-grade MDS (refractory anemia with excess of blasts). All diagnoses were assigned without the prior knowledge of JAK2 mutation testing results.
The JAK2 gene was successfully amplified by our real-time PCR melting curve assay in all 45 cases (examples shown in Fig. 1). The mutant allele was detected in 17 out of 18 (94%) patients with a MPN (according to the final diagnosis in Table 1), including five out of five cases of prefibrotic PMF, six out of six cases of PMF in the fibrotic stage, two out of two cases of AML transformed from preexisting PMF, one out of two cases of ET with fibrosis, and three out of three cases of PV with fibrosis (also termed post-polycythemic myeloid metaplasia, PPMM; Table 1). Only the wild-type allele was detected in 27 out of 27 (100%) patients with other neoplastic myeloid disorders associated with fibrosis, including 14 out of 14 cases of MDS-F, three out of three cases of ALL with marked fibrosis, four out of four cases of AML with mild to moderate fibrosis, two out of two cases of CMML with moderate fibrosis, one out of one case of CLL with mild fibrosis, one out of one case of large granular lymphocytic leukemia with moderate fibrosis, one out of one case of mastocytosis with marked fibrosis, and one out of one case of metastatic transitional cell carcinoma with marked fibrosis (Table 1).
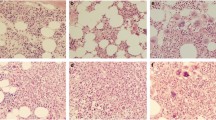
Of note, 19 cases (Table 1, cases 1–19) were originally descriptively diagnosed as “with features indeterminate for MDS versus MPN” due to insufficient clinicopathological evidence for definitive categorization. Figure 2 illustrates the significant overlapping features of MDS and MPN in two representative cases at initial presentation. One (panel A to C) was later determined to be prefibrotic PMF, and the other was reclassified as MDS-F with additional data obtained from follow-up biopsies and/or clinical findings. Importantly, JAK2 V617F mutant alleles were detected in all cases reclassified as PMF (Table 1, cases 1–5), whereas only JAK2 wild-type alleles were detected in all cases reclassified as MDS-F (Table 1, cases 6–19).
Microscopic evaluation of fibrotic bone marrow specimens. Representative micrographs of two cases with fibrotic marrow and “features indeterminate for MDS versus MPN” at the time of initial clinical presentation, that were later reclassified as PMF (a–c) and MDS-F (d–f). Low power (a and d, respectively), high power (b and e, respectively), and reticulin stains (c and f, respectively) are shown. Both cases demonstrate overlapping morphological features including hypercellularity, mild dysplasia and fibrosis, making a definitive diagnosis difficult. b demonstrates the increased bizarre megakaryocytes often observed in PMF, and e demonstrates the mononucleated megakaryocytes often seen in MDS. However, the morphologic evaluation alone as shown at initial presentation is not sufficient to render a specific diagnosis without follow-up biopsies and additional clinical findings
Discussion
Our results suggest that JAK2 V617F mutation screening is an important ancillary test for distinguishing between MDS and other BCR-ABL negative MPN with marrow fibrosis. Distinction between MPN and MDS is important for the appropriate clinical management of hematology patients. However, some cases may occasionally demonstrate overlapping morphological, laboratory, and clinical features that result in considerable diagnostic difficulty, particularly when the marrow aspirate is not optimal for morphologic evaluation due to the associated marrow fibrosis and when clinical data is limited or peripheral blood smears are unavailable for review. Our results demonstrated that JAK2 V617F was detected in nearly all cases of BCR-ABL negative MPN with fibrosis (Table 1). Only one case of JAK2 V617F-negative ET was identified. This particular patient may carry one of the other more rare JAK2 or MPL mutations not detected by our real-time PCR assay [26, 27]. In comparison, only wild-type alleles were detected in each case diagnosed as a non-MPN myeloid disorder with marrow fibrosis (Table 1). Other reports have recently demonstrated the occurrence of JAK2 V617F in MDS/MPN cases, including chronic myelomonocytic leukemia and atypical chronic myelogenous leukemia [28–30]. However, compared to MPN, a lower frequency of JAK2 mutations was identified. Taken together, analysis of the JAK2 mutation, in difficult-to-classify cases, will help to clarify the borderline between MDS (lacking JAK2 mutation) on one hand, and atypical MPN and MDS/MPN on the other hand. It would have been interesting to also include cases of autoimmune-associated marrow fibrosis, another setting that may mimic MPN or MDS associated with marrow fibrosis. However, no cases were found in our patient databases. Additionally, in our series, we found that acute leukemia arising in PMF retain the JAK2 V617F-positive genotype, whereas no JAK2 mutations were found in de novo AML with fibrosis. Since AML transformed from a preexisting MPN has a poorer overall prognosis, JAK2 genotyping may also have value in stratifying risk groups or predicting therapeutic responses in acute myeloid leukemia patients [31, 32].
Our observations suggest that JAK2 V617F testing is particularly helpful for cases with marrow fibrosis that are difficult to classify into MDS or MPN at the initial presentation [26]. Among the 19 specimens initially described as “with features indeterminate for MDS versus MPN”, JAK2 V617F mutant alleles were detected in each case that was ultimately reclassified as PMF (Table 1, cases 1–5), whereas only wild-type alleles were detected in the cases eventually reclassified as MDS-F (Table 1, cases 6–19). Although each patient was reclassified based on the evaluation of subsequent morphological and clinical information, JAK2 V617F genotyping could have substantially aided diagnosis at the time of initial presentation since the retrospective JAK2 V617F studies were concordant with the final disease phenotype. The JAK genotyping results, together with other clinical–morphological data and follow-up information, will be important for rendering a definitive diagnosis as early as possible during the disease course. This will emerge as an increasingly important capability when specific treatments targeting the JAK2 signaling pathway become available.
JAK2 V617F is not observed in well-defined MDS cases with marrow fibrosis in our cohort. This suggests that this mutation must play a minimal role, if any, in the fibrosis that occasionally occurs in MDS. However, it remains controversial whether JAK2 V617F is associated with MDS with fibrosis. Initially, a report by Ohyashiki et al., described the presence of JAK2 V617F in two of six MDS cases with fibrosis, but it was absent in multiple cases of AML, lymphoma, chronic myeloid leukemia with fibrosis, and MDS without fibrosis [33]. However, the results of our cohort and another study by Kremer et al. do not support that hypothesis [34]. In agreement with our findings, they also reported that JAK2 V617F is exceedingly rare in bona fide MDS or de novo AML, regardless of the presence or absence of fibrosis [34].
The optimal assays used to detect JAK2 V617F in the setting of marrow fibrosis should be highly sensitive and capable of using formalin-fixed materials as described here. Of note, although the markedly hemodiluted marrow smears and clot sections associated with bone marrow fibrosis are suboptimal for morphological evaluation, they contained a sufficient number of clonal hematopoietic cells for successful DNA extraction and molecular testing. In our laboratory, this real-time PCR melting curve assay was previously shown to have an analytical sensitivity of 5% (i.e., capable of detecting 5 JAK2 V617F-positive cells per 100 total cells) [20, 21]. This sensitivity may at least partially attribute to the higher percentages of PMF patients carrying JAK2 V617F in our cohort than reported in the literature [4–19]. Other explanations would include biased sampling in our cohort and different types of samples tested between our study (predominantly archived marrow samples) and other studies (predominantly using fresh blood samples). Additionally, the possibility of a case selection bias for JAK2 V617F screening in the cited studies cannot be excluded. Furthermore, at times, when additional marrow aspirate slides are not available, the ability to test for this mutation using formalin-fixed clot sections will eliminate the need for subsequent phlebotomies that can significantly prolong turnaround time.
In summary, our results demonstrate that JAK V617F is very frequently identified in fibrotic bone marrow specimens associated with BCR/ABL negative MPN, but it is not observed in MDS with marrow fibrosis. These findings indicate that the evaluation of JAK2 mutation status is an important tool that may aid the diagnosis of patients with marrow fibrosis.
References
Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, Barosi G, Verstovsek S, Birgegard G, Mesa R, Reilly JT, Gisslinger H, Vannucchi AM, Cervantes F, Finazzi G, Hoffman R, Gilliland DG, Bloomfield CD, Vardiman JW (2007) Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 110:1092–1097
Tefferi A, Vardiman JW (2007) The diagnostic interface between histology and molecular tests in myeloproliferative disorders. Current opinion in hematology 14:115–122
Rice L, Baker KR (2006) Current management of the myeloproliferative disorders: a case-based review. Arch Pathol Lab Med 130:1151–1156
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144–1148
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, White H, Zoi C, Loukopoulos D, Terpos E, Vervessou EC, Schultheis B, Emig M, Ernst T, Lengfelder E, Hehlmann R, Hochhaus A, Oscier D, Silver RT, Reiter A, Cross NC (2005) Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 106:2162–2168
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352:1779–1790
Passamonti F, Rumi E, Pietra D, Lazzarino M, Cazzola M (2007) JAK2 (V617F) mutation in healthy individuals. Br J Haematol 136:678–679
Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, Gilliland DG, Tefferi A (2005) JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 131:208–213
Levine RL, Loriaux M, Huntly BJ, Loh M, Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen K, Flores N, Estey E, Gattermann N, Armstrong S, Look TA, Griffin JD, Bernard OA, Gilliland DG, Druker BJ, Deininger MW (2005) The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106:3377–3379
Zhao ZJ, Vainchenker W, Krantz SB, Casadevall N, Constantinescu SN (2005) Role of tyrosine kinases and phosphatases in polycythemia vera. Semin Hematol 42:221–229
Tefferi A, Lasho TL, Schwager SM, Steensma DP, Mesa RA, Li CY, Wadleigh M, Gary Gilliland D (2005) The JAK2 tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol 131:320–328
Tefferi A, Sirhan S, Lasho TL, Schwager SM, Li CY, Dingli D, Wolanskyj AP, Steensma DP, Mesa R, Gilliland DG (2005) Concomitant neutrophil JAK2 mutation screening and PRV-1 expression analysis in myeloproliferative disorders and secondary polycythaemia. Br J Haematol 131:166–171
Johan MF, Goodeve AC, Bowen DT, Frew ME, Reilly JT (2005) JAK2 V617F Mutation is uncommon in chronic myelomonocytic leukaemia. Br J Haematol 130:968
Sulong S, Case M, Minto L, Wilkins B, Hall A, Irving J (2005) The V617F mutation in Jak2 is not found in childhood acute lymphoblastic leukaemia. Br J Haematol 130:964–965
Lasho TL, Mesa R, Gilliland DG, Tefferi A (2005) Mutation studies in CD3+, CD19+ and CD34+ cell fractions in myeloproliferative disorders with homozygous JAK2(V617F) in granulocytes. Br J Haematol 130:797–799
Ahmed A, Chang CC (2006) Chronic idiopathic myelofibrosis: clinicopathologic features, pathogenesis, and prognosis. Arch Pathol Lab Med 130:1133–1143
Cao M, Olsen RJ, Zu Y (2006) Polycythemia vera: new clinicopathologic perspectives. Arch Pathol Lab Med 130:1126–1132
Sanchez S, Ewton A (2006) Essential thrombocythemia: a review of diagnostic and pathologic features. Arch Pathol Lab Med 130:1144–1150
Olsen RJ, Tang Z, Farkas DH, Bernard DW, Zu Y, Chang CC (2006) Detection of the JAK2(V617F) mutation in myeloproliferative disorders by melting curve analysis using the LightCycler system. Arch Pathol Lab Med 130:997–1003
Hsieh P, Olsen RJ, O’Malley DP, Konoplev S, Hussong JW, Dunphy CH, Perkins SL, Cheng L, Lin P, Chang CC (2007) The role of Janus Kinase 2 V617F mutation in extramedullary hematopoiesis of the spleen in neoplastic myeloid disorders. Mod Pathol 20:929–935
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A (2006) European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90:1128–1132
Jaffe ES, Harris NL, Stein H, Vardiman JW (2001) Tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer (IARC), Lyon, France
Michiels JJ, Thiele J (2002) Clinical and pathological criteria for the diagnosis of essential thrombocythemia, polycythemia vera, and idiopathic myelofibrosis (agnogenic myeloid metaplasia). Int J Hematol 76:133–145
Steensma DP, Tefferi A (2003) The myelodysplastic syndrome(s): a perspective and review highlighting current controversies. Leuk Res 27:95–120
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison CN, Warren AJ, Gilliland DG, Lodish HF, Green AR (2007) JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 356:459–468
Mercher T, Wernig G, Moore SA, Levine RL, Gu TL, Frohling S, Cullen D, Polakiewicz RD, Bernard OA, Boggon TJ, Lee BH, Gilliland DG (2006) JAK2T875N is a novel activating mutation that results in myeloproliferative disease with features of megakaryoblastic leukemia in a murine bone marrow transplantation model. Blood 108:2770–2779
Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, Gilliland DG, Tefferi A (2005) The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood 106:1207–1209
Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, Beran M, Estey E, Kantarjian HM, Issa JP (2005) JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood 106:3370–3373
Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen KT, Flores NJ, Estey E, Gattermann N, Armstrong S, Look AT, Griffin JD, Bernard OA, Heinrich MC, Gilliland DG, Druker B, Deininger MW (2005) The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106:3377–3379
Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, Gugliotta L, Landolfi R, Kutti J, Gisslinger H, Marilus R, Patrono C, Pogliani EM, Randi ML, Villegas A, Tognoni G, Barbui T (2005) Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 105:2664–2670
Phekoo KJ, Richards MA, Moller H, Schey SA (2006) The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica 91:1400–1404
Ohyashiki K, Aota Y, Akahane D, Gotoh A, Miyazawa K, Kimura Y, Ohyashiki JH (2005) The JAK2 V617F tyrosine kinase mutation in myelodysplastic syndromes (MDS) developing myelofibrosis indicates the myeloproliferative nature in a subset of MDS patients. Leukemia 19:2359–2360
Kremer M, Horn T, Dechow T, Tzankov A, Quintanilla-Martinez L, Fend F (2006) The JAK2 V617F mutation occurs frequently in myelodysplastic/myeloproliferative diseases, but is absent in true myelodysplastic syndromes with fibrosis. Leukemia 20:1315–1316
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Olsen, R.J., Dunphy, C.H., O’Malley, D.P. et al. The implication of identifying JAK2 V617F in myeloproliferative neoplasms and myelodysplastic syndromes with bone marrow fibrosis. J Hematopathol 1, 111–117 (2008). https://doi.org/10.1007/s12308-008-0014-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-008-0014-8