Abstract
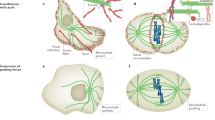
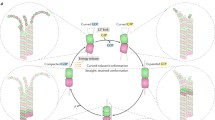
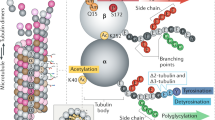
Microtubules are hollow tubes some 25 nm in diameter participating in the eukaryotic cytoskeleton. They are built from αβ-tubulin heterodimers that associate to form protofilaments running lengthwise along the microtubule wall with the β-tubulin subunit facing the microtubule plus end conferring a structural polarity. The α- and β-tubulins are highly conserved. A third member of the tubulin family, γ-tubulin, plays a role in microtubule nucleation and assembly. Other members of the tubulin family appear to be involved in microtubule nucleation. Microtubule assembly is accompanied by hydrolysis of GTP associated with β-tubulin so that microtubules consist principally of ‘GDP-tubulin’ stabilized at the plus end by a short ‘cap’. An important property of microtubules is dynamic instability characterized by growth randomly interrupted by pauses and shrinkage. Many proteins interact with microtubules within the cell and are involved in essential functions such as microtubule growth, stabilization, destabilization, and interactions with chromosomes during cell division. The motor proteins kinesin and dynein use microtubules as pathways for transport and are also involved in cell division. Crystallography and electron microscopy are providing a structural basis for understanding the interactions of microtubules with antimitotic drugs, with motor proteins and with plus end tracking proteins.






Similar content being viewed by others
References
Baldauf, S. L., Roger, A. J., Wenk-Siefert, I., & Doolittle, W. F. (2000). A kingdom level phylogeny of eukaryotes based on combined protein data. Science, 290, 972–977.
Luduena, R. F. (1998). Multiple forms of tubulin: Different gene products and covalent modifications. International Review of Cytology, 178, 207–275.
Dutcher, S. K. (2003). Long-lost relatives reappear: Identification of new members of the tubulin superfamily. Current Opinion in Microbiology, 6, 634–640.
Wilson, P. G., & Borisy, G. G. (1997). Evolution of the multi-tubulin hypothesis. BioEssays, 19, 451–454.
McKean, P. G., Vaughan, S., & Gull, K. (2001). The extended tubulin superfamily. Journal of Cell Science, 114, 2723–2733.
Savage, C., & Chalfie, M. (1991). Genetic aspects of microtubule biology in the nematode Caenorhabditis elegans. Cell Motility and the Cytoskeleton, 18, 159–163.
Fukushiga, T., Siddiqui, Z. K., Chou, M., Culotti, J. G., Gogonea, C. B., Siddiqui, S. S., et al. (1999). Mec-12, an α-tubulin required for touch sensitivity in C. elegans. Journal of Cell Science, 112, 395–403.
Verhey, K. J., & Gaertig, J. (2007). The tubulin code. Cell Cycle, 6, 2152–2160.
Hammond, J. W., Cai, D., & Verhey, K. J. (2008). Tubulin modifications and their cellular functions. Current Opinion in Cell Biology, 20, 71–76.
Fukushima, N., Furuta, D., Hidaka, Y., Moriyama, R., & Tsujiuchi, T. (2009). Posttranslational modifications of tubulin in the nervous system. Journal of Neurochemistry, 109, 683–693.
Oakely, C. E., & Oakely, B. R. (1989). Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature, 338, 662–664.
Oakely, B. R. (1992). γ-tubulin: The microtubule organiser? Trends in Cell Biology, 2, 1–5.
Kollman, J. M., Zelter, A., Muller, E. G., Fox, B., Rice, L. M., Davis, T. N., et al. (2008). The structure of the gamma-tubulin small complex: Implications of its architecture and flexibility for microtubule nucleation. Molecular Biology of the Cell, 19, 207–215.
Moritz, M., & Agard, D. A. (2001). Gamma-tubulin complexes and microtubule nucleation. Current Opinion in Structural Biology, 11, 174–181.
Dictenberg, J. B., Zimmerman, W., Sparks, C. A., Young, A., Vidair, C., Zheng, Y., et al. (1998). Pericentrin and γ-tubulin form a protein complex and are organised into a novel lattice at the centrosome. Journal of Cell Biology, 141, 163–174.
Zimmerman, W. C., Sillibourne, J., Rosa, J., & Doxsey, S. J. (2004). Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Molecular Biology of the Cell, 15, 3642–3657.
Goehring, N. W., & Beckwith, J. (2005). Diverse paths to midcell: Assembly of the bacterial cell division machinery. Current Biology, 15, R514–R526.
Erickson, H. P. (1997). FtsZ, a tubulin homologue in prokaryote cell division. Trends in Cell Biology, 7, 362–370.
Löwe, J., & Amos, L. A. (1998). Crystal structure of the bacterial cell division protein FtsZ. Nature, 391, 203–206.
Nogales, E., Downing, K. H., Amos, L. A., & Löwe, J. (1998). Tubulin and FtsZ form a distinct family of GTPases. Nature Structural Biology, 5, 451–458.
Oliva, M. A., Cordell, S. C., & Lowe, J. (2004). Structural insights into FtsZ protofilament formation. Nature Structural & Molecular Biology, 11, 1243–1250.
Haydon, D. J., Stokes, N. R., Ure, R., Galbraith, G., Bennett, J. M., Brown, D. R., et al. (2008). An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science, 321, 1673–1675.
Huang, Q., Tonge, P. J., Slayden, R. A., Kirikae, T., & Ojima, I. (2007). FtsZ: A novel target for tuberculosis drug discovery. Current Topics in Medicinal Chemistry, 7, 527–543.
Jenkins, C., Samudrala, R., Anderson, I., Hedlund, B. P., Petroni, G., Michailova, N., et al. (2002). Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter. Proceedings of the National Academy of Sciences of the United States of America, 99, 17049–17054.
Sontag, C. A., Staley, J. T., & Erickson, H. P. (2005). In vitro assembly and GTP hydrolysis by bacterial tubulins BtubA and BtubB. Journal of Cell Biology, 169, 233–238.
Pilhofer, M., Rosati, G., Ludwig, W., Schliefer, K.-H., & Petroni, G. (2007). Coexistence of tubulins and FtsZ in different Prosthecobacter species. Molecular Biology and Evolution, 24, 1439–1442.
Schlieper, D., Oliva, M. A., Andreu, J. M., & Löwe, J. (2005). Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer. Proceedings of the National Academy of Sciences of the United States of America, 102, 9170–9175.
Asnes, C. F., & Wilson, L. (1979). Isolation of bovine brain microtubule proteins without glycerol: Polymerisation kinetics change during purification cycles. Analytical Biochemistry, 98, 64–73.
Carlier, M.-F. (1991). Nucleotide hydrolysis in cytoskeletal assembly. Current Opinion in Cell Biology, 3, 12–17.
Caplow, M. (1992). Microtubule dynamics. Current Opinion in Cell Biology, 4, 58–65.
Chrétien, D., Fuller, S. D., & Karsenti, E. (1995). Structure of growing microtubule ends: Two-dimensional sheets close into tubes at variable rates. Journal of Cell Biology, 129, 1311–1328.
Aldaz, H., Rice, L. M., & Agard, D. A. (2005). Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature, 435, 523–527.
Rice, L. M., Montabana, E. A., & Agard, D. A. (2008). The lattice as allosteric effector: structural studies of alpha- beta- and gamma-tubulin clarify the role of GTP in microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America, 105, 5378–5383.
Dimitrov, A., Quesnoit, M., Moutel, S., Cantaloube, I., Poüs, C., & Perez, F. (2008). Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues. Science, 322, 1353–1356.
Hyman, A. A., Salser, S., Drechsel, D. N., Unwin, N., & Mitchison, T. J. (1992). Role of GTP hydrolysis in microtubule dynamics: Information from a slowly hydrolyzable analogue, GMPCPP. Molecular Biology of the Cell, 3, 1155–1167.
Mandelkow, E.-M., Mandelkow, E., & Milligan, R. A. (1991). Microtubule dynamics and microtubule caps: A time-resolved cryo-electron microscopy study. Journal of Cell Biology, 114, 977–991.
Melki, R., Carlier, M.-F., Pantaloni, D., & Timasheff, S. N. (1989). Cold depolymerization of microtubules to double rings: Geometric stabilization of assemblies. Biochemistry, 28, 9143–9152.
Horio, T., & Hotani, H. (1986). Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature, 321, 605–607.
Mitchison, T., & Kirschner, M. (1984). Dynamic instability of microtubule growth. Nature, 312, 237–242.
O’Connell, C. B., & Khodjakov, A. L. (2007). Cooperative mechanisms of mitotic spindle formation. Journal of Cell Science, 120, 1717–1722.
Severin, F. F., Sorger, P. K., & Hyman, A. A. (1997). Kinetochores distinguish GTP from GDP forms of the microtubule lattice. Nature, 388, 888–891.
Amos, L. A., & Klug, A. (1974). Arrangement of subunits in flagellar microtubules. Journal of Cell Science, 14, 523–549.
Raff, E. C., Fackenthal, J. D., Hutchens, J. A., Hoyle, H. D., & Turner, F. R. (1997). Microtubule architecture specified by a β-tubulin isoform. Science, 275, 70–73.
Chrétien, D., & Wade, R. H. (1991). New data on the microtubule surface lattice. Biology of the Cell, 71, 161–174.
Meurer-Grob, P., Kasparian, J., & Wade, R. H. (2001). Microtubule structure at improved resolution. Biochemistry, 40, 8000–8008.
Mitchison, T. J. (1993). Localisation of an exchangeable GTP binding site at the plus end of microtubules. Science, 261, 1044–1047.
Hirose, K., Fan, J., & Amos, L. A. (1995). Re-examination of the polarity of microtubules and sheets decorated with kinesin motor domain. Journal of Molecular Biology, 251, 329–333.
Fan, J., Griffith, A. D., Lockhart, A., & Cross, R. A. (1996). Microtubule minus ends can be labelled with a phage display antibody specific to alpha-tubulin. Journal of Molecular Biology, 259, 325–330.
Song, Y.-H., & Mandelkow, E. (1993). Recombinant kinesin motor domain binds to beta-tubulin and decorates microtubules with a B surface lattice. Proceedings of the National Academy of Sciences of the United States of America, 90, 1671–1675.
Metoz, F., Arnal, I., & Wade, R. H. (1997). Tomography without tilt: Three-dimensional imaging of microtubule-motor complexes. Journal of Structural Biology, 118, 159–168.
Wade, R. H., & Hyman, A. A. (1997). Microtubule structure and dynamics. Current Opinion in Cell Biology, 9, 12–17.
Nogales, E., Wolf, S. G., & Downing, K. A. (1998). Structure of the αβ-tubulin dimer by electron crystallography. Nature, 391, 199–203.
Gigant, B., Curmi, P. A., Martin-Barbey, C., Charbaut, E., Lachkar, S., Lebeau, L., et al. (2000). The 4 Å X-ray structure of a tubulin:stathmin-like domain complex. Cell, 102, 809–816.
Ravelli, R. B. G., Gigant, B., Curmi, P. A., Jourdain, I., Lachkar, S., Sobel, A., et al. (2004). Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature, 428, 198–202.
Gigant, B., Wang, C., Ravelli, R. B. G., Roussi, F., Steinmetz, M. O., Curmi, P. A., et al. (2005). Structural basis for the regulation of tubulin by vinblastine. Nature, 435, 519–522.
Li, H., DeRosier, D. J., Nicholson, W. V., Nogales, E., & Downing, K. H. (2002). Microtubule structure at 8 Å resolution. Structure, 10, 1317–1328.
Nogales, E., Whittaker, M., Milligan, R. A., & Downing, K. H. (1999). High resolution model of the microtubule. Cell, 96, 79–88.
Heinz, D. W., Schubert, W.-D., & Höfle, G. (2005). Much anticipated—the bioactive conformation of epothilone and its binding to tubulin. Angewandte Chemie (International ed. in English), 44, 1298–1301.
Nettles, J. H., Li, H., Cornett, B., Krahn, J. M., Snyder, J. P., & Downing, K. H. (2004). The binding mode of epothiline A on αβ-tubulin by electron crystallography. Science, 305, 866–869.
Hyman, A. A., Chrétien, D., Arnal, I., & Wade, R. H. (1995). Structural changes accompanying GTP hydrolysis in microtubules: Information from a slowly hydrolyzable analogue guanylyl-(a, b)-methylene-diphosphonate. Journal of Cell Biology, 128, 117–125.
Arnal, I., & Wade, R. H. (1995). How does taxol stabilize microtubules? Current Biology, 5, 900–905.
Elie-Caille, C., Severin, F., Helenius, J., Howard, J., Muller, D. J., & Hyman, A. A. (2007). Straight GDP-tubulin protofilaments form in the presence of taxol. Current Biology, 17, 1–6.
Ozon, S., Maucauer, A., & Sobel, A. (1997). The stathmin family—molecular and biological characterisation of novel mammalian proteins expressed in the nervous system. European Journal of Biochemistry, 248, 794–806.
Wood, K. W., Cornwell, W. D., & Jackson, J. R. (2001). Past and future of the mitotic spindle as an oncology target. Current Opinion in Pharmacology, 1, 370–377.
Fojo, T., & Menefee, M. (2007). Mechanisms of multidrug resistance: The potential role of microtubule stabilizing agents. Annals of Oncology, 18(Suppl 5), 3–8.
Morris, P. G., & Fornier, M. N. (2009). Novel anti-tubulin cytotoxic agents for breast cancer. Expert Review of Anticancer Therapy, 9, 175–185.
Kellogg, D. R., Field, C. M., & Alberts, B. M. (1989). Identification of microtubule-associated proteins in the centrosome, spindle, and kinetochore of the early Drosophila embryo. Cell Biology, 109, 2977–2991.
Sauer, G., Körner, R., Hanisch, A., Ries, A., Nigg, E. A., & Silljé, H. H. W. (2005). Proteome analysis of the human mitotic spindle. Molecular and Cellular Proteomics, 4, 35–43.
Hughes, J. R., Meireles, A. M., Fisher, K. H., Garcia, A., Antrobus, P. R., Wainman, A., et al. (2008). A microtubule interactome: Complexes with roles in cell cycle and mitosis. PLoS Biology, 6, e98.
Dehmelt, L., & Halpain, S. (2004). The MAP2/tau family of microtubules associated proteins. General Biology, 6, 204.
Baas, P. W., & Qiang, L. (2005). Neuronal microtubules: When the MAP is the roadblock. Trends in Cell Biology, 15, 183–187.
Al-Bassam, J., Ozer, R. S., Safer, D., Halpain, S., & Milligan, R. A. (2002). MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. Journal of Cell Biology, 157, 1187–1196.
Dixit, R., Ross, J. L., Golman, Y. E., & Holzbaur, E. L. F. (2008). Differential regulation of dynein and kinesin motor proteins by tau. Science, 319, 1086–1089.
Zhang, D., Rogers, G. C., Buster, D. W., & Sharp, D. J. (2007). Three microtubule severing enzymes contribute to the ‘Pacman-flux’ machinery that moves chromosomes. Journal of Cell Biology, 177, 231–242.
Stoppin-Mellet, V., Gaillard, J., Timmers, T., Neumann, E., Conway, J., & Vantard, M. (2007). Arabidopsis katanin binds microtubules using a multimeric microtubule-binding domain. Plant Physiology and Biochemistry, 45, 867–877.
Moore, J. D., & Endow, S. A. (1996). Kinesin proteins: A phylum of motors for microtubule-based motility. Bioessays, 18, 207–219.
Cole, D. G., & Scholey, J. M. (1995). Structural variations among the kinesins. Trends in Cell Biology, 5, 259–262.
Dagenbach, E. M., & Endow, S. A. (2004). A new kinesin tree. Journal of Cell Science, 117, 3–7.
Wickstead, B., & Gull, K. (2006). A ‘holistic’ kinesin phylogeny reveals new kinesin families and predicts protein functions. Molecular Biology of the Cell, 17, 1734–1743.
Kinesin Homepage. http://www.proweb.org/kinesin/.
Lawrence, J. L., et al. (2004). A standardized kinesin nomenclature. Journal of Cell Biology, 167, 19–22.
Fehr, A. N., Asbury, C. L., & Block, S. M. (2008). Kinesin steps do not alternate in size. Biophysical Journal, 94, L20–L22.
Verbrugge, S., Kapitein, L. C., & Peterman, E. J. (2007). Kinesin moving through the spotlight: Single-motor fluorescence microscopy with submillisecond time resolution. Biophysical Journal, 92, 2536–2545.
Hackney, D. D. (2007). Jump starting kinesin. Journal of Cell Biology, 176, 7–9.
Protein Data Bank, http://www.rcsb/pdb/.
Endow, S. A., & Waligora, K. W. (1998). Determinants of kinesin motor polarity. Science, 281, 1200–1202.
Wade, R. H., & Kozielski, F. (2000). Structural links to kinesin directionality and movement. Nature Structural Biology, 7, 456–460.
Kull, F. J., Sablin, A. P., Lau, R., Fletterick, R. J., & Vale, R. D. (1996). Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature, 380, 550–559.
Vallee, R. B., Williams, J. C., Varma, D., & Barnhart, L. E. (2003). Dynein: An ancient motor protein involved in multiple modes of transport. Journal of Neurobiology, 58, 189–200.
Wickstead, B., & Gull, K. (2007). Dyneins across eukaryotes: A comparative genomic analysis. Traffic, 8, 1708–1721.
King, S. M. (2000). AAA domains and the organisation of the dynein motor unit. Journal of Cell Science, 113, 2521–2526.
Carter, A. P., Garbarino, J. E., Wilson-Kubalek, E. M., Shipley, W. E., Cho, C., Milligan, R. A., et al. (2008). Structure and functional role of dynein’s microtubule-binding domain. Science, 322, 1691–1695.
Kon, T., Imamula, K., Roberts, A. J., Ohkura, R., Knight, P. J., Gibbons, I. R., et al. (2009). Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nature Structural & Molecular Biology, 16, 325–333.
Akhmanova, A., & Hoogenraad, C. C. (2005). Microtubule plus-end-tracking proteins: Mechanisms and functions. Current Opinion in Cell Biology, 17, 47–54.
Honnappa, S., Okhrimenko, O., Jaussi, R., Jawhari, H., Jelesarov, I., Winkler, F. K., et al. (2006). Key interaction modes of dynamic +TIP networks. Molecular Cell, 23, 663–671.
Morrison, E. E. (2007). Action and interactions at microtubule ends. Cellular and Molecular Life Sciences, 64, 307–317.
Slep, K. C., & Vale, R. D. (2007). Structural basis of microtubule plus end tracking by XMAP215, CLIP-170 and EB1. Molecular Cell, 27, 976–991.
Brouhard, G. J., Stear, J. H., Noetzel, T. L., Al-Bassam, J., Kinoshita, K., Harrison, S. C., et al. (2008). XMAP215 is a processive microtubule polymerase. Cell, 132, 79–88.
Vitre, B., Coquelle, F. M., Heichette, C., Garnier, C., Chrétien, D., & Arnal, I. (2008). EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nature Cell Biology, 10, 415–421.
Sandblad, L., Busch, K. E., Tittmann, P., Gross, H., Brunner, D., & Hoenger, A. (2006). The Schizosaccharomyces pombe EB1 homolog Mal3p binds and stabilizes the microtubule lattice seam. Cell, 127, 1415–1424.
des Georges, A., Katsuki, M., Drummond, D. R., Osei, M., Cross, R. A., & Amos, L. A. (2008). Mal3, the Schizosaccharomyces pombe homolog of EB1, changes the microtubule lattice. Nature Structural & Molecular Biology, 10, 1102–1108.
Korenbaum, E., & Rivero, F. (2002). Calponin homology domains at a glance. Journal of Cell Science, 115, 3543–3545.
Mishima, M., Maesaki, R., Kasa, M., Watanabe, T., Fukata, M., Kaibuchi, K., et al. (2007). Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition. Proceedings of the National Academy of Sciences of the United States of America, 104, 10346–10351.
Dragestein, K. A., van Cappellen, W. A., van Haren, J., Tsibidis, G. D., Akhamanova, A., Knock, T. A., et al. (2008). Dynamic behaviour of GFP-CLIP-170 reveals fast protein turnover on microtubules plus ends. Journal of Cell Biology, 180, 729–737.
Beiling, P., Kandels-Lewis, S., Telley, I. A., van Djik, J., Janke, C., & Surrey, T. (2008). CLIP-170 tracks growing microtubule ends by dynamically recognising composite EB1/tubulin binding sites. Journal of Cell Biology, 183, 1223–1233.
Dixit, R., Barnett, B., Lazarus, J. B., Tokito, M., Goldman, Y. E., & Holzbaur, E. L. (2009). Microtubule plus-end tracking by CLIP-170 requires EB1. Proceedings of the National Academy of Sciences of the United States of America, 106, 492–497.
Peris, L., Thery, M., Fauré, J., Saoudi, Y., Lafanachère, L., Chilton, J. K., et al. (2006). Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. Journal of Cell Biology, 174, 839–849.
Weisbrich, A., Honnappa, S., Jaussi, R., Okhrimenko, O., Frey, D., Jelesarov, I., et al. (2007). Structure-function relationship of CAP-Gly domains. Nature Structural & Molecular Biology, 14, 959–967.
Watson, P., & Stephens, D. J. (2006). Microtubule plus-end loading of p150Glued is mediated by EB1 and CLIP-170 but is not required for intracellular membrane traffic in mammalian cells. Journal of Cell Science, 119, 2758–2767.
Cheeseman, I. M., & Desai, A. (2008). Molecular architecture of the kinetochore-microtubule interface. Nature Reviews. Molecular Cell Biology, 9, 33–46.
Tanaka, T. U., & Desai, A. (2008). Kinetochore-microtubule interactions: The means to the end. Current Opinion in Cell Biology, 20, 53–63.
Wilson-Kubalek, E. M., Cheeseman, I. M., Yoshioka, C., Desai, A., & Milligan, R. A. (2008). Orientation and structure of the Ndc80 complex on the microtubule lattice. Journal of Cell Biology, 182, 1055–1061.
Ciferri, C., Pasqualato, S., Screpanti, E., Varetti, G., Santaguida, S., Dos Reis, G., et al. (2008). Implications for kinetochore-microtubule attachment from the structure of an engineered Ncd80 complex. Cell, 133, 427–439.
Howard, J., & Hyman, A. A. (2007). Microtubule polymerases and depolymerases. Current Opinion in Cell Biology, 19, 31–35.
Mayr, M. I., Hümmer, S., Bormann, J., Grüner, T., Adio, S., Woehlke, G., et al. (2007). The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Current Biology, 17, 488–498.
Tanenbaum, M. E., Galijart, N., van Vugt, M. A., & Medema, R. H. (2006). CLIP-170 facilitates the formation of kinetochore-microtubule attachments. EMBO Journal, 25, 45–57.
Scholey, J. M. (2009). Kinesin-5 in Drosophila embryo mitosis: Sliding filament or spindle matrix mechanism? Cell motility and the cytoskeleton. epub www.interscience.wiley.com.
Gardner, M. K., & Odde, D. J. (2008). Dam1 complexes go it alone on disassembling microtubules. Nature Cell Biology, 10, 379–381.
Davis, T. N., & Wordman, L. (2007). Rings, bracelets, sleeves and chevrons: New structures of kinetochore proteins. Trends in Cell Biology, 17, 377–382.
Varga, V., Helenius, J., Tanaka, K., Hyman, A. A., Tanaka, T. U., & Howard, J. (2006). Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature Cell Biology, 8, 957–962.
Gardner, M. K., Odde, D. J., & Bloom, K. J. (2008). Kinesin-8 molecular motors: Putting the brakes on chromosome oscillations. Trends in Cell Biology, 18, 307–310.
McIntosh, J. R., Grischuk, E. L., Morphew, M. K., Efremov, A. K., Zhudenkov, K., Volkov, V. A., et al. (2008). Fibrils connect microtubule tips with kinetochore: A mechanism to couple tubulin dynamics to chromosome movement. Cell, 135, 322–333.
Wade, R. H., Meurer-Grob, P., Metoz, F., & Arnal, I. (1998). Organisation and structure of microtubules and microtubule-motor protein complexes. European Biophysics Journal, 27, 446–454.
Acknowledgments
Many thanks to Isabelle Arnal, Isabel Garcia-Saez, Didier Job and Dimitrios Skoufias for critically reading the manuscript and for all their remarks and corrections. Needless to say, remaining mistakes are all mine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wade, R.H. On and Around Microtubules: An Overview. Mol Biotechnol 43, 177–191 (2009). https://doi.org/10.1007/s12033-009-9193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-009-9193-5