Abstract
Introduction
The Na+–K+–2Cl− cotransporter localized in the brain vascular endothelium has been shown to be important in the evolution of cerebral edema following experimental stroke. Previous in vivo studies have demonstrated that bumetanide, a selective Na+–K+–2Cl− cotransport inhibitor, attenuates ischemia-evoked cerebral edema. Recently, bumetanide has been shown to also inhibit water permeability via aquaporin-4 (AQP4) expressed in Xenopus laevis oocytes. We tested the hypothesis that the perivascular pool of AQP4 plays a significant role in the anti-edema effect of bumetanide by utilizing wild-type (WT) mice as well as mice with targeted disruption of α-syntrophin (α-Syn−/−) that lack the perivascular pool of AQP4.
Methods
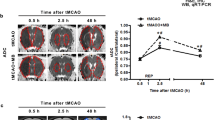
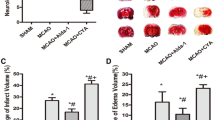
Isoflurane-anesthetized adult male WT C57Bl6 and α-Syn−/− mice were subjected to 90 min middle cerebral artery occlusion (MCAO) followed by 24 or 48 h of reperfusion. Adequacy of MCAO and reperfusion was monitored with laser-Doppler flowmetry over the ipsilateral parietal cortex. Infarct volume (tetrazolium staining), cerebral edema (wet-to-dry ratios), and AQP4 protein expression (immunoblotting) were determined in different treatment groups in separate sets of experiments.
Results
Bumetanide significantly attenuated infarct volume and decreased ipsilateral hemispheric water content in WT mice compared to vehicle treatment. In α-Syn−/− mice, bumetanide treatment had no effect on infarct volume or ischemia-evoked cerebral edema. Bumetanide-treated WT mice had a significant attenuation of AQP4 protein expression at 48 h post-MCAO compared to vehicle-treated WT mice.
Conclusions
These data suggest that bumetanide exerts its neuroprotective and anti-edema effects partly via blockade of the perivascular pool of AQP4 and may have therapeutic potential for ischemic stroke in the clinical setting.




Similar content being viewed by others
References
Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci. 2002;9:113–24.
Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (malignant) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–3.
Klatzo I. Neuropathological aspects of cerebral edema. J Neuropathol Exp Neurol. 1967;26:1–14.
Bhardwaj A. Osmotherapy in neurocritical care. Curr Neurol Neurosci Rep. 2007;7:513–21.
Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–9.
Menzies SA, Betz AL, Hoff JT. Contributions of ions and albumin to the formation and resolution of ischemic brain edema. J Neurosurg. 1993;78:257–66.
Schielke GP, Moises HC, Betz AL. Blood to brain sodium transport and interstitial fluid potassium concentration during focal ischemia in the rat. J Cereb Blood Flow Metab. 1991;11:466–71.
Zeynalov E, Chen CH, Froehner SC, Adams ME, Ottersen OP, Amiry-Moghaddam M, Bhardwaj A. The perivascular pool of aquaporin-4 mediates the effect of osmotherapy in postischemic cerebral edema. Crit Care Med. 2008;36:2634–40.
King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–48.
Manley GT, Fujimura M, Ma T, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–63.
Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–78.
Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stødkilde-Jørgensen H, Sulyok E, Dóczi T, Neely JD, Agre P, Frøkiaer J, Nielsen S. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci USA. 2002;99:13131–6.
Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An α-syntrophin dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA. 2003;100:2106–11.
Amiry-Moghaddam M, Xue R, Haug F-M, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18:542–4.
Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–3.
Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108:2993–3002.
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80.
Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–13.
Plotkin MD, Cummings BS, Grant DF, Schnellmann RG. Expression of the Na+–K+–2Cl− cotransporter BSC2 in the nervous system. Am J Physiol. 1997;272:C173–83.
O’Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na–K–Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046–56.
O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na–K–Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26:1234–49.
Yuen N, Anderson SE, Glaser N, Tancredi DJ, O’Donnell ME. Cerebral blood flow and cerebral edema in rats with diabetic ketoacidosis. Diabetes. 2008;57:2588–94.
O’Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na–K–Cl cotransporter activity is V1 receptor- and [Ca]-dependent. Am J Physiol Cell Physiol. 2005;289:C283–92.
Foroutan S, Brillault J, Forbush B, O’Donnell ME. Moderate to severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na–K–Cl cotransporter. Am J Physiol Cell Physiol. 2005;289:C1492–501.
Yan Y, Dempsey RJ, Flemmer A, Forbush B, Sun D. Inhibition of Na+–K+–Cl− cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003;961:22–31.
Migliati ER, Meurice N, Dubois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool AJ. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol Pharm. 2009;76:105–12.
Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182, 780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–8.
Liu X, Zhang W, Alkayed NJ, Adams ME, Amiry-Moghaddam M, Ottersen OP, Hurn PD, Bhardwaj A. Lack of sex-linked differences in cerebral edema and aquaporin-4 expression after experimental stroke. J Cereb Blood Flow Metab. 2008;28:1898–906.
Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–98.
Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–70.
Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–11.
Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21.
Chen C-H, Toung TJK, Sapirstein A, Bhardwaj A. Effect of duration of osmotherapy on blood–brain barrier disruption and regional cerebral edema after experimental stroke. J Cereb Blood Flow and Metab. 2006;26:951–8.
Toung TJ, Chen CH, Lin C, Bhardwaj A. Osmotherapy with hypertonic saline attenuates water content in brain and extracerebral organs. Crit Care Med. 2007;35:526–31.
Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca(2 +)/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci. 1999;19:7823–33.
Kako K, Wakamatsu H, Hamada T, Banasik M, Ohata K, Niki-Kuroiwa T, Suzuki S, Takeuchi J, Ishida N. Examination of DNA-binding activity of neuronal transcription factors by electrophoretical mobility shift assay. Brain Res Protocols. 1998;2:243–9.
Yan Y, Dempsey RJ, Sun D. Na+–K+–Cl− cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:711–21.
Haas M. The Na–K–Cl cotransporters. Am J Physiol. 1994;267:C869–85.
Haas M, McManus TJ. Bumetanide inhibits (Na+–K+–2Cl−) co-transport at a chloride site. Am J Physiol. 1983;245:C235–40.
Suvitayavat W, Palfrey HC, Haas M, Rao MC. Characterization of the endogenous Na(+)–K(+)–2Cl-cotransporter in Xenopus oocytes. Am J Physiol. 1984;266:C284–92.
Kumar V, Naik RS, Hillert M, Klein J. Effects of chloride flux modulators in an in vitro model of brain edema formation. Brain Res. 2006;1122:222–9.
McClain RM, Dammers KD. Toxicology evaluation of bumetanide, potent diuretic agent. J Clin Pharmacol. 1981;21:543–54.
Frydenlund DS, Bhardwaj A, Otsuka T, Mylonakou MN, Yasumura T, Davidson KG, Zeynalov E, Skare O, Laake P, Haug FM, Rash JE, Agre P, Ottersen OP, Amiry-Moghaddam M. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci USA. 2006;103:13532–6.
MacAulay N, Hamann S, Zeuthen T. Water transport in the brain: role of cotransporters. Neuroscience. 2004;129:1031–44.
Hamann S, Herrera-Perez JJ, Bundgaard M, Alvarez-Leefmans FJ, Zeuthen T. Water permeability of Na+–K+–2Cl− cotransporters in mammalian epithelial cells. J Physiol. 2005;568:123–35.
Acknowledgments
This work was supported by Public Health Service NIH grants NS046379 (AB) and NS33145 (SCF, MEA). The authors thank Stepahnie J. Murphy, D.V.M., Ph.D., and Sarah Mader for maintaining the colony for transgenic mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migliati, E.R., Amiry-Moghaddam, M., Froehner, S.C. et al. Na+–K+–2Cl− Cotransport Inhibitor Attenuates Cerebral Edema Following Experimental Stroke via the Perivascular Pool of Aquaporin-4. Neurocrit Care 13, 123–131 (2010). https://doi.org/10.1007/s12028-010-9376-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9376-8