Abstract
Background and purpose
Animals subjected to an inflammatory insult with lipopolysaccharide (LPS) at the time of stroke are predisposed to develop a detrimental autoimmune response to myelin basic protein (MBP). In this study, we sought to determine whether other inflammatory stimuli could similarly invoke central nervous system (CNS) autoimmunity and whether these detrimental autoimmune responses occurred to antigens other than MBP.
Methods
Male Lewis rats underwent 3 h middle cerebral artery occlusion (MCAO) and received intraperitoneal injections of LPS, staphylococcal enterotoxin B (SEB), lipoteichoic acid (LTA) or saline at the time of reperfusion. Behavioral tests were performed at set time intervals after MCAO and animals were sacrificed at 1 month to analyze the immune response to MBP, neuron specific enolase (NSE) and proteolipid protein (PLP).
Results
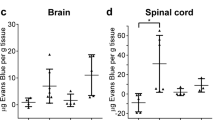
Lymphocytes from SEB treated animals were highly reactive to all tested CNS antigens, but treatment with LPS was most likely to lead to a Th1(+) response. A Th1(+) response to MBP, NSE or PLP in spleen was associated with worse outcome, although the response to NSE was most predictive of poor outcome. Animals with a cell mediated autoimmune response to either MBP or NSE in spleen had a concomitant humoral response to these antigens.
Conclusions
These data show that LPS, but not other inflammatory stimuli, increase the likelihood of developing a detrimental autoimmune response to an array of brain antigens.








Similar content being viewed by others
References
Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75:749–55.
Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–44.
Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40.
Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–20.
Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–14.
Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53.
Harms H, Prass K, Meisel C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. 2008;3:e2158.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Huang W, Koller LD. Superantigen activation and kinetics of cytokines in the Long-Evans rat. Immunology. 1998;95:331–8.
Chatterjee PK, Zacharowski K, Cuzzocrea S, et al. Lipoteichoic acid from Staphylococcus aureus reduces renal ischemia/reperfusion injury. Kidney Int. 2002;62:1249–63.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6.
Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–24.
Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–15.
Becker KJ. Sensitization and tolerization to brain antigens in stroke. Neuroscience. 2009;158:1090–7.
Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16:23–6.
Toubi E, Shoenfeld Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity. 2004;37:183–8.
Huang YH, Haegerstrand A, Frostegard J. Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin Exp Immunol. 1996;103:61–6.
Croft M, Dubey C. Accessory molecule and costimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118.
Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2.
Kato H, Kogure K, Liu XH, Araki T, Itoyama Y. Progressive expression of immunomolecules on activated microglia and invading leukocytes following focal cerebral ischemia in the rat. Brain Res. 1996;734:203–12.
Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–40.
Jiang-Shieh YF, Yeh KY, Wei IH, et al. Responses of microglia in vitro to the gram-positive bacterial component, lipoteichoic acid. J Neurosci Res. 2005;82:515–24.
Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1:181–91.
Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–4.
Manicassamy S, Ravindran R, Deng J, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–9.
Kerfoot SM, Long EM, Hickey MJ, et al. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol. 2004;173:7070–7.
Kato N, Fujii Y, Agata N, et al. Experimental murine model for autoimmune myocarditis using Klebsiella pneumoniae O3 lipopolysaccharide as a potent immunological adjuvant. Autoimmunity. 1993;14:231–6.
Zaccone P, Fehervari Z, Blanchard L, Nicoletti F, Edwards CK 3rd, Cooke A. Autoimmune thyroid disease induced by thyroglobulin and lipopolysaccharide is inhibited by soluble TNF receptor type I. Eur J Immunol. 2002;32:1021–8.
Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol. 2003;133:299–306.
Friedman SM, Tumang JR, Crow MK. Microbial superantigens as etiopathogenic agents in autoimmunity. Rheum Dis Clin North Am. 1993;19:207–22.
Ivars F. Superantigen-induced regulatory T cells in vivo. Chem Immunol Allergy. 2007;93:137–60.
Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–66.
Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–82.
Hofstetter HH, Targoni OS, Karulin AY, Forsthuber TG, Tary-Lehmann M, Lehmann PV. Does the frequency and avidity spectrum of the neuroantigen-specific T cells in the blood mirror the autoimmune process in the central nervous system of mice undergoing experimental allergic encephalomyelitis? J Immunol. 2005;174:4598–605.
Muhallab S, Lidman O, Weissert R, Olsson T, Svenningsson A. Intra-CNS activation by antigen-specific T lymphocytes in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;113:202–11.
Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–62.
Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–30.
Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–62.
Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2006;172:187–97.
Fillit HM, Kemeny E, Luine V, Weksler ME, Zabriskie JB. Antivascular antibodies in the sera of patients with senile dementia of the Alzheimer’s type. J Gerontol. 1987;42:180–4.
Jankovic BD, Horvat J, Djordjijevic D, Ramah A, Fridman V, Spahic O. Brain-associated autoimmune features in heroin addicts: correlation to HIV infection and dementia. Int J Neurosci. 1991;58:113–26.
Braus BK, Hauck SM, Amann B, et al. Neuron-specific enolase antibodies in patients with sudden acquired retinal degeneration syndrome. Vet Immunol Immunopathol. 2008;124:177–83.
Ikeda Y, Maruyama I, Nakazawa M, Ohguro H. Clinical significance of serum antibody against neuron-specific enolase in glaucoma patients. Jpn J Ophthalmol. 2002;46:13–7.
Jankovic BD, Djordjijevic D. Differential appearance of autoantibodies to human brain S100 protein, neuron specific enolase and myelin basic protein in psychiatric patients. Int J Neurosci. 1991;60:119–27.
Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–64.
Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30.
Umehara H, Bloom E, Okazaki T, Domae N, Imai T. Fractalkine and vascular injury. Trends Immunol. 2001;22:602–7.
Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87.
Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15:1663–8.
Soriano SG, Amaravadi LS, Wang YF, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65.
Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–21.
Kastenbauer S, Koedel U, Wick M, Kieseier BC, Hartung HP, Pfister HW. CSF and serum levels of soluble fractalkine (CX3CL1) in inflammatory diseases of the nervous system. J Neuroimmunol. 2003;137:210–7.
Matsunawa M, Isozaki T, Odai T, et al. Increased serum levels of soluble fractalkine (CX3CL1) correlate with disease activity in rheumatoid vasculitis. Arthritis Rheum. 2006;54:3408–16.
Yajima N, Kasama T, Isozaki T, et al. Elevated levels of soluble fractalkine in active systemic lupus erythematosus: potential involvement in neuropsychiatric manifestations. Arthritis Rheum. 2005;52:1670–5.
Fraticelli P, Sironi M, Bianchi G, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–81.
Acknowledgment
This work was supported by grants from the National Institutes of Neurological Disorders and Stroke (NINDS) (1RO1NS056457).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zierath, D., Thullbery, M., Hadwin, J. et al. CNS Immune Responses Following Experimental Stroke. Neurocrit Care 12, 274–284 (2010). https://doi.org/10.1007/s12028-009-9270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-009-9270-4