Abstract
Background
Although antibiotic-loaded spacers are commonly used to treat periprosthetic infections, it is unclear whether spacers continue to release bactericidal levels of antibiotic 6 weeks after implantation.
Questions/purposes
We asked whether an antibiotic can be detected in the tissue surrounding the spacer 6 weeks after implantation and whether the concentration is higher than the minimal inhibition concentration (MIC) previously determined for pathogens that are responsible for most periprosthetic infections.
Methods
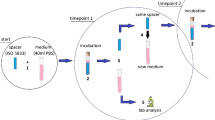
We removed 14 spacers used in two-stage septic revisions of infected hip prostheses 6 weeks after the primary implantations and determined the concentration of the antibiotics in the membrane formed between the spacer and the neighboring bone on the acetabular and the femoral sides. In seven cases Copal cement with gentamicin and clindamycin were used, and in seven other cases vancomycin was added to the Copal cement. Concentrations of the spacer antibiotics in the neighboring tissue were determined by tandem mass spectroscopy.
Results
All three antibiotics were detected in concentrations higher than their MIC. There were no differences between the groups regardless whether vancomycin was added to the cement, or whether the cement was applied with the acetabular cup spacer or with the stem spacer.
Conclusions
We concluded that, using the spacer technique described in this study, 6 weeks after spacer implantation, the concentrations of antibiotic are sufficient to treat a periprosthetic infection.
Similar content being viewed by others
References
Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol. 1998;36:2932–2939.
Baleani M, Persson C, Zolezzi C, Andollina A, Borelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23:1232–1238.
Bertazonni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53:329–334.
Bunetel L. Segui A, Cormier M, Percheron E, Langlais F. Release of gentamicin form acrylic bone cement. Clin Pharmacokinet. 1989;17:291–297.
Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882.
Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466:1492–1498.
Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46.
Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty. 1999;14:175–181.
Fink B. Revision of late periprosthetic infections of total hip endoprostheses: pros and cons of different concepts. Int J Med Sci. 2009;6:287–295.
Fink B, Grossmann A, Fuerst M, Schäfer P, Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467:1848–1858.
Fink B, Rechtenbach A, Büchner H, Vogt S, Hahn M. Articulating spacers used in two-stage revision of infected hip and knee prostheses abrade with time. Clin Orthop Relat Res. 2010 Jul 28. [Epub ahead of print].
Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem. 1953;203:359–366.
Garvin KL, Evans BG, Salvati EA, Brause BD. 1994. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Relat Res. 1994;298:97–105.
Garvin KL, Hanssen AD. Current concepts review: infection after total hip arthroplasty. J Bone Joint Surg Am. 1995;77:1576–1588.
Gonzales Della Valle A, Bostrom M, Brause B, Harney C, Salvati EA. Effective bactericidal activity of tobramycin and vancomycin eluted form acrylic bone cement. Acta Orthop Scand. 2001;72:237–240.
Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694.
Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85.
Heller DN, Peggins JO, Nochetto CB, Smith ML, Chiesa OA, Moulton K. LC/MS/MS measurement of gentamicine in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J Chromatogr B. 2005;821:22–30.
Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879.
Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res. 2006;24:1615–1621.
Hsieh PH, Shih CH, Chang YH, Lee MS, Yang WE, Shih HN. Treatment of deep infection of the hip associated with massive bone loss. Two-stage revision with an antibiotic-loaded interim cement prosthesis followed by reconstruction with allograft. J Bone Joint Surg Br. 2005;87:770–775.
Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005;441:243–249.
Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two staged reimplantation protocol. Clin Orthop Relat Res. 1994;301:205–212.
Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics form bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty 1998;13:331–338.
Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72–78.
Mitchell HH, Hamilton TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem. 1945;158:625–637.
Neut D, de Groot EP, Kowalski RSZ, van Horn JR, van der Mei HC, Busscher HJ. Gentamicin-loaded bone cement with clindamycin and fusidic acid added: biofilm formation and antibiotic release. J Biomed Mater Res A. 2005;73:165–170.
Pandey R, Drakouilakis E, Athanasou NA. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52:118–123.
Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939–944.
Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–1409.
Simpson PM, Dall GF, Breusch SJ, Heisel C. In vitro elution and mechanical properties of antibiotic-loaded SmartSet HV and Palocor R acrylic bone cements. Orthopäde. 2005;34:1255–1262.
Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res. 2005;23:27–33.
Törholm C, Lindgren L, Kahlmoter G. Total hip joint arthroplasty with gentamicin-impregnated cement: a clinical study of gentamicin excretion kinetics. Clin Orthop Relat Res. 1983;181:99–106.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Guidance for industry: bioanalytical method validation. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed May 2, 2011.
Virolainen P, Lahteenmaki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg. 2002;91:178–181.
Wahlig H, Dingeldein E. Buchholz HW, Buchholz M, Bachmann F. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements: comparative effects of varying dosage. J Bone Joint Surg Br. 1984;66:175–179.
Acknowledgments
We thank Lars Frommelt MD, Service for Infectious Diseases, Clinical Microbiology and Infection Control, ENDO-Klinik, Hamburg, Germany, for support in choosing the local and systemic antibiotic therapy for the patients of this study, and in writing this paper. We also thank D. Labes, Biostatistics, Cooperative Clinical Drug Research and Development, Neuenhagen, Germany, for support in performing the statistics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Orthopaedic Clinic Markgröningen gGmbH, Markgröningen, Germany.
About this article
Cite this article
Fink, B., Vogt, S., Reinsch, M. et al. Sufficient Release of Antibiotic by a Spacer 6 Weeks after Implantation in Two-stage Revision of Infected Hip Prostheses. Clin Orthop Relat Res 469, 3141–3147 (2011). https://doi.org/10.1007/s11999-011-1937-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-1937-4