Abstract
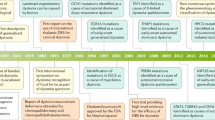
The dystonias comprise a group of syndromes characterized by prolonged involuntary muscle contractions resulting in repetitive movements and abnormal postures. Primary dystonia has been associated with over 14 different genotypes, most of which follow an autosomal dominant inheritance pattern with reduced penetrance. Independent of etiology, the disease is characterized by extensive variability in disease phenotype and clinical severity. Recent neuroimaging studies investigating this phenomenon in manifesting and non-manifesting genetic carriers of dystonia have discovered microstructural integrity differences in the cerebello-thalamo-cortical tract in both groups related to disease penetrance. Further study suggests these differences to be specific to subrolandic white matter regions somatotopically related to clinical phenotype. Clinical severity was correlated to the degree of microstructural change. These findings suggest a mechanism for the penetrance and clinical variability observed in dystonia and may represent a novel therapeutic target for patients with refractory limb symptoms.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34.
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–73.
Kamm C. Early onset torsion dystonia (Oppenheim’s dystonia). Orphanet J Rare Dis. 2006;1:48.
Nemeth AH. The genetics of primary dystonias and related disorders. Brain. 2002;125:695–721.
Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136:2017–37.
Moghimi N, Jabbari B, Szekely AM. Primary dystonias and genetic disorders with dystonia as clinical feature of the disease. Eur J Paediatr Neurol. 2013. doi:10.1016/j.ejpn.2013.05.015.
Bressman SB. Dystonia genotypes, phenotypes, and classification. In: Fahn S, Hallett M, DeLong MR, editors. Dystonia 4: advances in neurology. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 101–7.
Bressman S. Genetics of dystonia. J Neural Transm Suppl. 2006:489–95.
Risch NJ, Bressman SB, Senthil G, Ozelius LJ. Intragenic Cis and Trans modification of genetic susceptibility in DYT1 torsion dystonia. Am J Hum Genet. 2007;80:1188–93.
Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, et al. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A(Part A):2098–105.
Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609.
Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–32.
Dobricic VS, Kresojevic ND, Svetel MV, Jankovic MZ, Petrovic IN, Tomic AD, et al. Mutation screening of the DYT6/THAP1 gene in Serbian patients with primary dystonia. J Neurol. 2013;260:1037–42.
Houlden H, Schneider SA, Paudel R, Melchers A, Schwingenschuh P, Edwards M, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–50.
Xiromerisiou G, Houlden H, Scarmeas N, Stamelou M, Kara E, Hardy J, et al. THAP1 mutations and dystonia phenotypes: genotype phenotype correlations. Mov Disord. 2012;27:1290–4.
Newman JR, Lehn AC, Boyle RS, Silburn PA, Mellick GD. Screening for rare sequence variants in the THAP1 gene in a primary dystonia cohort. Mov Disord. 2013. doi:10.1002/mds.25479.
Lohmann K, Klein C. Genetics of dystonia: What’s known? What’s new? What’s next? Mov Disord. 2013;28:899–905.
Kaiser FJ, Osmanoric A, Rakovic A, Erogullari A, Uflacker N, Braunholz D, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6). Ann Neurol. 2010;68:554–9.
Gavarini S, Cayrol C, Fuchs T, Lyons N, Ehrlich ME, Girard JP, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–53.
Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124:1269–76.
Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: evidence from neuroimaging. Neurobiol Dis. 2011;42:202–9.
Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–212.
Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(Pt 2):463–83.
Pettigrew LC, Jankovic J. Hemidystonia: a report of 22 patients and a review of the literature. J Neurol Neurosurg Psychiatry. 1985;48:650–7.
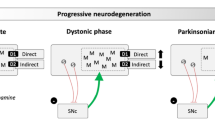
Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17 Suppl 3:S49–62.
Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201.
Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia - help or hindrance? Clin Neurophysiol. 2012;123:65–70.
•• Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. This study identifies neuropathological changes such as reduced Purkinje cell linear density in post-mortem human brains and links these changes to THAP1 sequence variations.
Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–51.
Blood AJ, Kuster JK, Woodman SC, Kirlic N, Makhlouf ML, Multhaupt-Buell TJ, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PloS One. 2012;7:e31654.
Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–9.
Ramdhani RA, Simonyan K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (NY). 2013;3:1–11.
Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28:944–57.
Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80.
Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–509.
Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–33.
LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–9.
• Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol Dis. 2012;49C:200–10. This study provides evidence of cerebellar dysfunction as the primary cause of dystonia symptoms.
Fan X, Hughes KE, Jinnah HA, Hess EJ. Selective and sustained alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation in cerebellum induces dystonia in mice. J Pharmacol Exp Ther. 2012;340:733–41.
Zhao Y, Sharma N, LeDoux MS. The DYT1 carrier state increases energy demand in the olivocerebellar network. Neuroscience. 2011;177:183–94.
Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage. 2011;56:1011–20.
Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Arch Neurol. 2009;66:502–8.
Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–6.
Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov Disord. 2008;23:234–9.
van der Meer JN, Beukers RJ, van der Salm SM, Caan MW, Tijssen MA, Nederveen AJ. White matter abnormalities in gene-positive myoclonus-dystonia. Mov Disord. 2012;27:1666–72.
Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol. 2008;15:185–9.
Bonilha L, de Vries PM, Hurd MW, Rorden C, Morgan PS, Besenski N, et al. Disrupted thalamic prefrontal pathways in patients with idiopathic dystonia. Parkinsonism Relat Disord. 2009;15:64–7.
Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. J Neurol Neurosurg Psychiatry. 2005;76:1591–3.
Bonilha L, de Vries PM, Vincent DJ, Rorden C, Morgan PS, Hurd MW, et al. Structural white matter abnormalities in patients with idiopathic dystonia. Mov Disord. 2007;22:1110–6.
Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–54.
Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–7.
Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Mol Pharmacol. 1999;55:23–31.
Erickson MA, Haburcak M, Smukler L, Dunlap K. Altered functional expression of Purkinje cell calcium channels precedes motor dysfunction in tottering mice. Neuroscience. 2007;150:547–55.
LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120:302–10.
Wilson BK, Hess EJ. Animal models for dystonia. Mov Disord. 2013;28:982–9.
•• Ulug AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, et al. Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc Natl Acad Sci U S A. 2011;108:6638–43. This study demonstrates evidence of cerebellothalamocortical dysfunction in DYT1 KI mice, similar to that as observed in human studies.
• Song CH, Fan X, Exeter CJ, Hess EJ, Jinnah HA. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2012;48:66–78. This study identifies microstructural abnormalities in the striatum of DYT1 KI mouse models, providing a correlate to changes observed in imaging studies.
Song CH, Bernhard D, Bolarinwa C, Hess EJ, Smith Y, Jinnah HA. Subtle microstructural changes of the striatum in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2013;54:362–71.
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–9.
Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–9.
Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5:320–30.
Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28:958–67.
Sadnicka A, Kimmich O, Pisarek C, Ruge D, Galea J, Kassavetis P, et al. Pallidal stimulation for cervical dystonia does not correct abnormal temporal discrimination. Mov Disord. 2013. doi:10.1002/mds.25581.
Toda H, Hamani C, Lozano A. Deep brain stimulation in the treatment of dyskinesia and dystonia. Neurosurg Focus. 2004;17:E2.
Acknowledgment
David Eidelberg has received grant support from the National Institute of Neurological Disorders and Stroke (R01 NS 072514), The Bachmann-Strauss Dystonia and Parkinson Foundation, High Q Foundation, and the Dana Foundation.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Renata Lerner has received an MD/PhD candidate stipend from Hofstra North Shore-LIJ School of Medicine. Martin Niethammer is employed by The Feinstein Institute for Medical Research. David Eidelberg has board membership with The Bachmann-Strauss Dystonia and Parkinson Foundation, Michael J. Fox Foundation for Parkinson’s Research, Thomas Hartman Foundation for Parkinson’s Research, Inc.; has been a consultant for Pfizer, Inc.; and is employed by The Feinstein Institute for Medical Research. He is coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same; no financial gain. He has received honoraria from The Bachmann-Strauss Dystonia and Parkinson Foundation and Michael J. Fox Foundation for Parkinson’s Research.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Lerner, R.P., Niethammer, M. & Eidelberg, D. Understanding the Anatomy of Dystonia: Determinants of Penetrance and Phenotype. Curr Neurol Neurosci Rep 13, 401 (2013). https://doi.org/10.1007/s11910-013-0401-0
Published:
DOI: https://doi.org/10.1007/s11910-013-0401-0