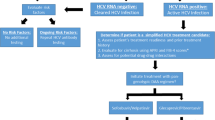
Abstract
The anticipation of all-oral therapy for hepatitis C virus (HCV) infection creates new opportunities for preventing HCV-related morbidity and mortality. This curative therapy is associated with fewer side effects and contraindications, which likely will greatly expand the number of persons eligible to receive treatment. To ensure that benefits from all-oral treatment regimens are realized for individuals and communities, public health can deliver essential services to meet its three fundamental roles: assessment, policy development, and assurance. Public health monitors HCV transmission and disease to prioritize populations needing care, and collaborates community-wide to create policies, plans, and programs to improve access to HCV testing and linkages to care. Public health also evaluates the effectiveness, accessibility, and quality of HCV care and treatment. Public health research can reveal how curative treatments can improve prevention and eliminate disparities. The extent to which public health can identify and link HCV-infected persons to quality care and appropriate therapy will be a key determining factor in whether the full benefits of curative HCV therapy are realized.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jacobson I, McHutchison J, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16.
Poordad F, McCone J, Bacon B, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–37.
Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16.
McGowan CE, Monis A, Bacon BR, et al. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatology. 2013 Apr;57(4):1325–32.
Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013 Mar;207 Suppl 1:S19–25.
McGowan CE, FriedMW. Barriers to hepatitis C treatment. Liver Int. 2012 Feb;32 Suppl 1:151–6.
North CS, Hong BA, Adewuyi SA, et al. Hepatitis C treatment and SVR: the gap between clinical trials and real-world treatment aspirations. Gen Hosp Psychiatry. 2013;35(2):122–8.
•• Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–61. This report estimates the proportion of HCV infected persons in the United States who have been tested, received care and completed anti-viral therapy.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87.
Poordad F, Lawitz E, Kowdley KV, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368(1):45–53.
Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;16;368(20):1907–17.
Martin NK, Vickerman P, Grebely J, et al.: HCV treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013 [Epub ahead of print].
Durier N, Nguyen C, White LJ. Treatment of hepatitis C as prevention: a modeling case study in Vietnam. PLoS One. 2012;7(4):e34548.
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2362–8.
Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14.
Chak E, Talal A, Sherman K, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–101.
Kim WR, Terrault NA, Pedersen RA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137(5):1680–6.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–73.
Davis KL, Mitra D, Medjedovic J, et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45(2):17–24.
Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012 Feb 21;156(4):263–70.
Ly K, Xing J, Klevens M, et al. The growing burden of mortality from viral hepatitis in the US, 1999–2007. Ann Intern Med. 2012;156(4):271.
Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–82.
Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(Suppl):62S–5.
Smith BD, Patel N, Beckett GA, et al.: Hepatitis C virus antibody prevalence, correlates and predictors among persons born from 1945 through 1965, United States, 1999–2008. [abstract] American Association for the Study of Liver Disease, San Francisco (CA)2011.
CDC: 2011 surveillance summary in press
CDC. Hepatitis C virus infection among adolescents and young adults –Massachusetts, 2002—2009. MMWR. 2011;60(17):537–54.
Garg S, Taylor LE, Grasso C, Mayer KH. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis. 2013;56(10):1480–7.
CDC. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005–2010. MMWR. 2011;60(28):945–50.
Institute of Medicine. Committee for the Study of the Future of Public Health, Division of Health Care Services: The future of public health. Washington, DC: National Academy Press; 1988.
Tsui JI, Maselli J, Gonzales R. Sociodemographic trends in national ambulatory care visits for hepatitis C virus infection. Dig Dis Sci. 2009;54(12):2694–8.
CDC. Hepatocellular carcinoma - United States, 2001–2006. MMWR. 2010;7;59(17):517–20.
CDC. Testing for HCV infection: An Update of Guidance for Clinicians and Laboratorians. MMWR. 2013;62(18):362–5.
Kim AY, Nagami EH, Birch CE, Bowen MJ, Lauer GM, McGovern BH. A simple strategy to identify acute hepatitis C virus infection among newly incarcerated injection drug users. Hepatology. 2013;57(3):944–52.
Macalino GE, Vlahov D, Dickinson BP, Schwartzapfel B, Rich JD. Community incidence of hepatitis B and C among reincarcerated women. Clin Infect Dis. 2005 Oct 1;41(7):998–1002.
•• U.S. Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care, and treatment of viral hepatitis. Washington, DC: HHS; 2011. p. p. 1–76. Prompted by a 2010 IOM report on the state of viral hepatitis prevention in the United States, this report outlines a comprehensive approach to viral hepatitis prevention that includes strategies to improve HCV education, testing, care and treatment, surveillance, and delivery of services to populations at risk. Many action steps within this plan have been undertaken by CDC and other HHS agencies, and additional actions will soon be put forth in an updated 2014–2016 viral hepatitis action plan.
•• Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010. In this report, IOM identified viral hepatitis as an “underappreciated health concern for the nation” and recommended ways for the federal government to improve prevention of HCV transmission and disease. This report raised awareness of the silent epidemic of viral hepatitis in the United States, outlining expectations for public health policy and spurring development of a U.S. viral hepatitis action plan.
•• CDC. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR. 2012;61(No. RR-04)):1–18. This CDC report provides expanded recommendations for HCV testing based on evidence that persons born during 1945–1965 represent 81% of all persons living with HCV and three quarters of HCV-associated mortality. The U.S. Preventive Services Task Force has issued similar recommendations. These recommendations will facilitate implementation of both risk-based and birth-cohort-based strategies for HCV testing and linkage to care and treatment.
Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56(1):40–50.
CDC. Characteristics of persons with chronic hepatic tis B—San Francisco, California, 2006. MMWR. 2007;56(18):446–8.
Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011 Jun 9;364(23):2199–207.
Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505.
Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57.
Ruark A, Shelton JD, Halperin DT, et al. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1078.
Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7(2):151–6.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. John W. Ward declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ward, J.W. The Role of Public Health in an Era of All-Oral Therapy for Hepatitis C Infection. Curr Hepatitis Rep 12, 220–226 (2013). https://doi.org/10.1007/s11901-013-0184-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-013-0184-4