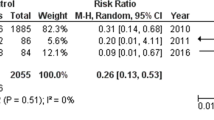
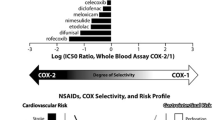
Abstract
There is convincing evidence that chemoprevention has the potential to be a major component of colorectal cancer control. Experimental, epidemiologic, and clinical studies provide evidence that nonsteroidal anti-inflammatory drugs (NSAIDs), particularly the selective cyclooxygenase (COX)-2 inhibitors, including celecoxib and several phytochemicals, act as anticancer agents. However, several of these chemopreventive agents induce side effects at effective high dose levels. Low doses of atorvastatin and aspirin, or atorvastatin and celecoxib, or piroxicam and difluoromethylornithine administered in combination are more effective in inhibiting chemically induced colon adenocarcinomas in male F 344 rats than are high doses of these agents given individually.
Similar content being viewed by others
References and Recommended Reading
Jamal A, Murray T, Ward E, Samuels A, et al.: Cancer statistics. CA Cancer J Clin 2005, 55:10–30.
World Cancer Research Fund and American Institute for Cancer Research: Panel on Food, Nutrition and Prevention. Washington DC: American Institute for Cancer Research; 1997.
Caygil CPJ, Charland SL, Lippin JA: Fat, fish and fish oil, and cancer. Br J Cancer 1996, 74:159–164.
Reddy BS: Omega-3 fatty acids in colorectal cancer prevention. Int J Cancer 2004, 112:1–7.
Rao CV, Hirose Y, Cooma I, Reddy BS: Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res 2001, 61:1927–1933.
Reddy BS, Maruyama H: Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F 344 rats. Cancer Res 1986, 46:3367–3370.
Thun MJ, Namboodiri MM, Heath CJ Jr: Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991, 325:1593–1596.
Thun MJ, Namboodiri MM, Calle EE, et al.: Aspirin use and risk of fatal cancer. Cancer Res 1993, 53:1322–1327.
Giovannucci E, Rimm EB, Stampfer M, et al.: Aspirin use and risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 1994, 121:241–246.
Kune G, Kune S, Watson LF: Colorectal cancer risk, chronic ilnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res 1988, 48:4399–4404.
Giardiello FM, Hamilton SR, Krush AJ, et al.: Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993, 328:1313–1316.
Labayle D, Fischer D, Vielh P, et al.: Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 1991, 101:635–639.
Lynch HT, Thorson AG, Smyrk T: Rectal cancer after prolonged sulindac chemoprevention: a case report. Cancer 1995, 75:936–938.
Tonelli F, Valanzano R: Sulindac in familial adenomatous polyposis. Lancet 1993, 342:1120.
Ladenheim J, Garcia G, Titzer D, et al.: Effect of sulindac on sporadic colonic polyps. Gastroenterology 1995, 108:1083–1087.
Reddy BS: Carcinogen-induced colon cancer models for chemoprevention and nutritional studies. In Tumor Models in Cancer Research. Edited by Teicher BA. Totowa, NJ: Human Press; 2002:183–191.
Narisawa T, Sato M, Tani M, et al.: Inhibition of development of methylnitrosourea-induced rat colon tumors by indomethacin treatment. Cancer Res 1981, 41:1954–1957.
Narisawa T, Sato M, Sano M, Takahashi T: Inhibition of development of methylnitrosourea-induced rat colonic tumors by peroral administration of indomethacin. Gann 1982, 73:377–381.
Pollard M, Luckert PH: Prolonged anti-tumor effect of indomethacin on autochthonous intestinal tumors in rats. J Natl Cancer Inst 1983, 70:1103–1105.
Reddy BS, Maruyama H, Kelloff G: Dose-related inhibition of colon carcinogenesis by dietary piroxicam: a nonsteroidal anti-inflammatory drug, during different stages of rat colon tumor development. Cancer Res 1987, 47:5340–5346.
Reddy BS, Tokumo K, Kulkarni N, et al.: Inhibition of colon carcinogenesis by prostaglandin synthesis inhibitors and related compounds. Carcinogenesis 1992, 13:1019–1023.
Reddy BS, Rao CV, Rivenson A, Kelloff G: Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F 344 rats. Carcinogenesis 1993, 14:1493–1497.
Moorghen M, Ince P, Finney KJ, et al.: The effect of sulindac on colonic tumor formation in dimethylhydrazine-treated mice. Acta Histochem 1990, 39(Suppl):195–199.
Skinner SA, Penney AG, O’Brien P: Sulindac inhibits the rate of growth and appearance of colon tumors in the rat. Arch Surg 1991, 126:1094–1096.
Kawamori T, Rao CV, Seibert K, Reddy BS: Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 1998, 58:409–412.
Reddy BS, Kawamori T, Lubet RA, et al.: Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer Res 1999, 59:3387–3391.
Wargovich MJ, Chen CD, Harris C, et al.: Inhibition of aberrant crypt growth by non-steroidal anti-inflammatory agents and differentiation agents in the rat colon. Int J Cancer 1995, 60:515–519.
Reddy BS, Rao CV, Seibert K: Evaluation of Cyclooxygenase-2 inhibitor for potential chemopreventative properties in colon carcinogenesis. Cancer Res 1996, 56:4566–4569.
Takahashi M, Fukutake M, Yokota S, et al.: Suppression of azoxymethane-induced aberrant crypt foci in rat colon by nimesulide, a selective inhibitor of cyclooxygenase 2. J Cancer Res Clin Oncol 1996, 122:219–222.
Reddy BS, Hirose Y, Lubet R, et al.: Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res 2000, 60:293–297.
Boolbol SK, Dannenberg AJ, Chadburn A, et al.: Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 1996, 56:2556–2560.
Chiu CH, Mcentee MF, Whelan J: Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosynthesis. Cancer Res 1997, 57:4267–4273.
Mahmoud NN, Dannenberg AJ, Mestre J, et al.: Aspirin prevents tumors in a murine model of familial adenomatous polyposis. Surgery 1998, 124:225–231.
Oshima M, Murai N, Kargman S, et al.: Chemoprevention of intestinal polyposis in the APC (Delta)716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res 2001, 61:1733–1740.
Reddy BS, Rao CV: Colon cancers: a role for cyclooxygenase-2 specific non-steroidal anti-inflammatory drugs. Drugs Aging 2000, 16:329–334.
Jacoby RF, Seibert K, Cole CF, et al.: The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the Min mouse model of adenomatous polyposis. Cancer Res 2000, 60:5040–5044. The results of this study clearly demonstrate that celecoxib is effective for the prevention of intestinal adenomas in the mouse model of adenomatous polyposis.
Williams JL, Borgo S, Hasan I, et al.: Nitric-oxide releasing NSAIDs alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer prevention. Cancer Res 2001, 61:3285–3289.
Rao CV, Simi B, Cooma I, et al.: Chemoprevention of colonic aberrant crypt foci by nitric oxide (NO)-releasing NSAIDs [abstract]. Front Cancer Prev Res 2002, 1:D321.
Cooma I, Malisetty VS, Patlolla JM, et al.: Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F 344 rats and modulation of Inos and COX-2, and apoptosis in human colon Ht-29 cancer cells [abstract]. Am Assoc Cancer Res 2002, 40:819.
Rao CV, Rivenson A, Simi B, Reddy BS: Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 1995, 55:259–266.
Kawamori T, Lubet R, Steele VE, et al.: Chemopreventive effect of curcumin, a naturally-occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 1999, 59:597–601.
Rao CV, Simi B, Reddy BS: Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci in the rat colon. Carcinogenesis 1993, 14:2219–2225.
Rao CV, Desai D, Rivenson A, et al.: Chemoprevention of colon carcinogenesis by phenlethyl-3-methylcaffeate. Cancer Res 1995, 55:2310–2315.
Rao CV, Desai D, Simi B, et al.: Inhibitory effects of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res 1993, 53:4182–4188.
Huang MT, Ma W, Yen P, et al.: Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in Hela cells. Carcinogenesis 1996, 17:761–765.
Mahmoud NN, Carothers AM, Grunberger D, et al.: Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis 2000, 21:921–927.
Xu YX, Pindolia KR, Janakiraman N, et al.: Curcumin Inhibits 11-1alpha and TNF-alpha induction of AP-1 and NF-kappaB DNA-binding activity in bone marrow stromal cells. Hematophathol Mol Hematol 1998, 11:49–62.
Taylor JD: Lipoxygenase regulation of membrane expression of tumor cell glycoproteins and subsequent metastasis. Adv Prostaglandin Thromboxane Leukot Res 1989, 19:439–443.
Natarajan K, Singh S, Burke TR Jr, et al.: Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor Nf-KappaB. 1996, 93:9090–9095.
Reddy BS, Rao CV: Chemoprevention of colon cancer by thiol and other organosulfur compounds. Phytochemicals in Cancer Prevention. Washington, DC: American Chemical Society; 1994, 546:164–172.
Reddy BS, Nayani J, Tokumo K, et al.: Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a non-steroidal anti-inflammatory drug with D, L-alphadifluoromethy-ornithine: an ornithine decarboxylase inhibitor, in diet. Cancer Res 1990, 50:2562–2568. This study evaluated the efficacy of combination of low doses of chemopreventive agents against colon carcinogenesis. The results of this study provided convincing evidence that administration of low doses of piroxicam, an NSAID, and difluoromethylornithine, an ornithine decarboxylase inhibitor, in combination are more effective in inhibiting colon carcinogenesis in F 344 rats than these agents administered alone at high dose levels.
Agarwal B, Rao CV, Bhendwal S, et al.: Lovastatin augments sulindac-induced apoptosis in colon cancer cells and potentiates chemopreventive effect of sulindac. Gastroenterology 1999, 117:838–847.
Malisetty VS, Cooma I, Reddy BS, et al.: Caspase-3-activity, and apoptosis induction by a combination of HMG-Coa reductase inhibitor and COX-2 inhibitors: a novel approach in developing effective chemopreventive regimens. Int J Oncol 2002, 20:753–759. The findings of this study indicate that HMG Co-A reductase and COX-2 activities play important roles in apoptosis and regulation of apoptosis by lovastatin and celecoxib and provide effective strategies for the prevention of colon cancer.
Li H, Schut HA, Conran P, et al.: Prevention by aspirin and its combination with alpha-difluoromethylornithine of azoxymethane-induced tumors, aberrant crypt foci and prostaglandin E2 levels in rat colon. Carcinogenesis 1999, 20:425–430.
Reddy BS, Wang CX, Steele VG, et al.: Synergistic effects of the combination of low doses of aspirin or celecoxib with lipitor against colon carcinogenesis: a promising chemoprevention strategy [Abstract]. Presented at the 96th Annual Meeting of the American Association for Cancer Research. April 16–20, 2005. This study concluded that administration of high doses of atorvastatin, celecoxib, and aspirin individually and low doses of these agents in combination in the diet significantly inhibited chemically induced colon adenocarcinomas in a realistic rat model. Low doses of atorvastatin and celecoxib and atorvastatin and aspirin in combination are more effective than the high doses of these agents given individually.
Reddy BS, Patlolla JM, Simi B, et al.: Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase as inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res 2005, in press. The results strongly indicate that very low, low, and moderately low levels of celecoxib administered in diets containing saturated fats or omega-3 fatty acids (ω-3 PUFAs) suppressed colon carcinogenesis. More importantly, the inhibition of colon adenocarcinomas was more pronounced in animals given very low doses of celecoxib in ω-3 PUFAs as compared with the effects of very low dose celecoxib given in a saturated fat diet.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reddy, B.S., Rao, C.V. Chemoprophylaxis of colon cancer. Curr Gastroenterol Rep 7, 389–395 (2005). https://doi.org/10.1007/s11894-005-0009-x
Issue Date:
DOI: https://doi.org/10.1007/s11894-005-0009-x