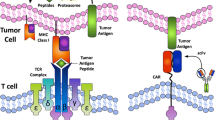
Abstract
Despite recent advances in the diagnosis and treatment of colorectal cancer, it remains the second commonest cause of cancer deaths in the USA. Current therapeutic agents have provided a small, incremental survival benefit at the cost of significantly increased toxicities. There is a huge unmet need for novel effective therapies. Colorectal cancer evades the host immune surveillance as a result of weak immunogenicity and immunosuppressive effects of cancer cells. Cancer vaccines have the potential to activate the immune system against colorectal cancer cells by increasing the expression and presentation of tumor-associated antigens. Recent clinical trials using viral-vector-based cancer vaccines have demonstrated clinical benefit with excellent safety profiles in patients with metastatic colorectal cancer. Here, we review the rationale for immunotherapy in colon cancer; clinical trial data for viral-vector-based colorectal cancer vaccines, the advantages and disadvantages of viral vectors, and future treatment strategies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Ferlay J et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Cheng L et al. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34(6):573–80.
Grothey A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Van Cutsem E et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506.
Van Cutsem E et al. Cetuximab plus Irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89.
Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Hoover Jr HC et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol. 1993;11(3):390–9.
Dunn GP et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Vesely MD et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.
•• Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: Recent progress and prospects. Immunol Cell Biol. 2013. doi:10.1038/icb.2013.29. The authors review myeloid-derived suppressor cells and regulatory T cells as potential cancer immunotherapy.
Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306.
Fernandez NC et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–11.
Kadowaki N et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193(10):1221–6.
Salaun B, Romero P, Lebecque S. Toll-like receptors' two-edged sword: When immunity meets apoptosis. Eur J Immunol. 2007;37(12):3311–8.
Kass E et al. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59(3):676–83.
Brown M et al. Antigen gene transfer to cultured human dendritic cells using recombinant avipoxvirus vectors. Cancer Gene Ther. 1999;6(3):238–45.
Sutter G, Staib C. Vaccinia vectors as candidate vaccines: The development of modified vaccinia virus Ankara for antigen delivery. Curr Drug Targets Infect Disord. 2003;3(3):263–71.
Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–56.
Marshall JL et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18(23):3964–73.
•• Morse MA et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother. 2013;62(8):1293–301. The authors show that the novel adenovirus subtype 5 (E1 - , E2b - ) gene delivery platform generates significant cell-mediated immune responses to the tumor antigen CEA in the setting of both naturally acquired and immunization-induced adenovirus subtype 5 specific immunity.
Holyoke D, Reynoso G, Chu TM. Carcinoembryonic antigen (CEA) in patients with carcinoma of the digestive tract. Ann Surg. 1972;176(4):559–64.
Kantor J et al. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84(14):1084–91.
Kaufman H, Schlom J, Kantor J. A recombinant vaccinia virus expressing human carcinoembryonic antigen (CEA). Int J Cancer. 1991;48(6):900–7.
Kantor J et al. Immunogenicity and safety of a recombinant vaccinia virus vaccine expressing the carcinoembryonic antigen gene in a nonhuman primate. Cancer Res. 1992;52(24):6917–25.
McAneny D et al. Results of a phase I trial of a recombinant vaccinia virus that expresses carcinoembryonic antigen in patients with advanced colorectal cancer. Ann Surg Oncol. 1996;3(5):495–500.
Conry RM et al. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5(9):2330–7.
Marshall JL et al. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol. 1999;17(1):332–7.
Hodge JW et al. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15(6–7):759–68.
Jager E et al. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer. 1996;67(1):54–62.
McLaughlin JP et al. Improved immunotherapy of a recombinant carcinoembryonic antigen vaccinia vaccine when given in combination with interleukin-2. Cancer Res. 1996;56(10):2361–7.
Hodge JW et al. Admixture of a recombinant vaccinia virus containing the gene for the costimulatory molecule B7 and a recombinant vaccinia virus containing a tumor-associated antigen gene results in enhanced specific T-cell responses and antitumor immunity. Cancer Res. 1995;55(16):598–603.
von Mehren M et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6(6):2219–28.
Hodge JW et al. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9(5):1837–49.
Marshall JL et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23(4):720–31.
Salazar E et al. Agonist peptide from a cytotoxic t-lymphocyte epitope of human carcinoembryonic antigen stimulates production of tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. Int J Cancer. 2000;85(6):829–38.
Bai XF et al. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111(10):1487–96.
Tsang KY et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11(4):1597–607.
Gulley JL et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–9.
•• Morse MA et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013. doi:10.1097/SLA.0b013e318292919e. The authors show that dendritic cells and pox vector vaccines have similar activity.
Harrop R et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: A phase I/II trial. Clin Cancer Res. 2006;12(11 Pt 1):3416–24.
MacDonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74(2):914–22.
Osada T et al. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and antitumor effects. Cancer Immunol Immunother. 2012;61(11):1941–51.
•• Lee J, Park YS, Burke J, Lim HY, Lee J, Kang WK, et al. Phase Ib dose-escalation study of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, administered by intravenous (IV) infusions in patients with metastatic colorectal carcinoma (mCRC). J Clin Oncol. 2013;31(15):3608. The authors show that repeated intravenous administration of Pexa-Vec was well tolerated, and antitumor activity was described in refractory colorectal cancer patients.
Kaufman HL et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008;14(15):4843–9.
Harrop R et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57(7):977–86.
Chakraborty M, Schlom J, Hodge JW. The combined activation of positive costimulatory signals with modulation of a negative costimulatory signal for the enhancement of vaccine-mediated T-cell responses. Cancer Immunol Immunother. 2007;56(9):1471–84.
Hodge JW et al. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174(10):5994–6004.
Iwai Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7.
Hirano F et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–96.
Palena C, Schlom J. Vaccines against human carcinomas: Strategies to improve antitumor immune responses. J Biomed Biotechnol. 2010;2010:380697.
Mosca PJ et al. Current status of dendritic cell immunotherapy of malignancies. Int Rev Immunol. 2003;22(3–4):255–81.
Morse MA et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11(8):3017–24.
•• Schlom J. Therapeutic cancer vaccines: Current status and moving forward. J Natl Cancer Inst. 2012;104(8):599–613. The author reviews cancer vaccine platforms that target a diverse range of TAAs that are currently being evaluated in randomized phase II and phase III trials.
Stein WD et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17(4):907–17.
Wolchok JD et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20.
Hoos A et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–97.
Prete SP et al. Combined effects of 5-fluorouracil, folinic acid and Oxaliplatin on the expression of carcinoembryonic antigen in human colon cancer cells: Pharmacological basis to develop an active antitumor immunochemotherapy. J Exp Clin Cancer Res. 2008;27:5.
Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–44.
Emens LA et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–8.
Morse MA. Technology evaluation: Ipilimumab, medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7(6):588–97.
Weber J. Review: Anti-CTLA-4 antibody ipilimumab: Case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864–72.
Madan RA et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–8.
Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13(6):847–61.
Compliance with Ethics Guidelines
Conflict of Interest
Noman Ashraf, Amit Mahipal, and Richard Kim declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
No funding has been received for preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashraf, N., Mahipal, A. & Kim, R. Viral Vector Vaccines To Treat Colorectal Cancer. Curr Colorectal Cancer Rep 9, 398–405 (2013). https://doi.org/10.1007/s11888-013-0185-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-013-0185-2