Abstract
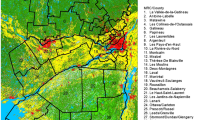
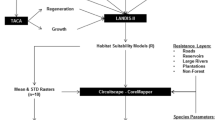
Biologic control of the introduced and invasive, woody plant tamarisk (Tamarix spp, saltcedar) in south-western states is controversial because it affects habitat of the federally endangered South-western Willow Flycatcher (Empidonax traillii extimus). These songbirds sometimes nest in tamarisk where floodplain-level invasion replaces native habitats. Biologic control, with the saltcedar leaf beetle (Diorhabda elongate), began along the Virgin River, Utah, in 2006, enhancing the need for comprehensive understanding of the tamarisk-flycatcher relationship. We used maximum entropy (Maxent) modeling to separately quantify the current extent of dense tamarisk habitat (>50% cover) and the potential extent of habitat available for E. traillii extimus within the studied watersheds. We used transformations of 2008 Landsat Thematic Mapper images and a digital elevation model as environmental input variables. Maxent models performed well for the flycatcher and tamarisk with Area Under the ROC Curve (AUC) values of 0.960 and 0.982, respectively. Classification of thresholds and comparison of the two Maxent outputs indicated moderate spatial overlap between predicted suitable habitat for E. traillii extimus and predicted locations with dense tamarisk stands, where flycatcher habitat will potentially change flycatcher habitats. Dense tamarisk habitat comprised 500 km2 within the study area, of which 11.4% was also modeled as potential habitat for E. traillii extimus. Potential habitat modeled for the flycatcher constituted 190 km2, of which 30.7% also contained dense tamarisk habitat. Results showed that both native vegetation and dense tamarisk habitats exist in the study area and that most tamarisk infestations do not contain characteristics that satisfy the habitat requirements of E. traillii extimus. Based on this study, effective biologic control of Tamarix spp. may, in the short term, reduce suitable habitat available to E. traillii extimus, but also has the potential in the long term to increase suitable habitat if appropriate mixes of native woody vegetation replace tamarisk in biocontrol areas.
Similar content being viewed by others
References
Bateman H L, Dudley T L, Bean D W, Ostoja S M, Hultine K R, Kuehn M J (2010). A river system to watch: Documenting the effects of saltcedar (Tamarix spp.) biocontrol in the Virgin River Valley. Ecol Res, 28(4): 405–410
Chapin F S III, Zavaleta E S, Eviner V T, Naylor R L, Vitousek P M, Reynolds H L, Hooper D U, Lavorel S, Sala O E, Hobbie S E, Mack M C, Díaz S (2000). Consequences of changing biodiversity. Nature, 405(6783): 234–242
Cory J S, Myers J H (2000). Direct and indirect ecological effects of biological control. Trends Ecol Evol, 15(4): 137–139
Deloach C J, Carruthers R, Dudley T, Eberts D, Kazmer D (2004). First results for control of saltcedar (Tamarix spp.) in the open field in the Western United States. In: Cullen, J M, Briese D T, Kriticos D J, Lonsdale W M, Morin L, Scott J K, eds. Proceedings of the XI International Symposium on Biological Control Weeds. Canberra, Australia: CSIRO Entomology, 505–513
Di Tomaso J M (1998). Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol, 12: 326–336
Dudley T L, DeLoach C J (2004). Saltcedar (Tamarix spp.), endangered species, and biological weed control — can they mix? Weed Technol, 18(sp1): 1542–1551
Durst S L, Sogge M K, Stump S D, Williams S O, Kus B E, Sferra S J (2007). Southwestern Willow Flycatcher breeding site and territory summary — 2006. USGS Open File Report 2007-1391
Elith J, Graham C H (2009). Do they / How do they / Why do they differ? — On finding reasons for differing performances of species distribution models. Ecography, 32(1): 66–77
Elith J, Graham C H, Anderson R P, Dudík M, Ferrier S, Guisan A, Hijmans R J, Huettmann F, Leathwick J R, Lehmann A (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29: 129–151
ESRI ArcGIS 9.2 (2006). Redlands, CA, USA, available online: www.esri.com
Evangelista P H, Stohlgren T J, Morisette J T, Kumar S (2009). Mapping invasive tamarisk (Tamarix): A comparison of single-scene and timeseries analyses of remotely sensed data. Remote Sens, 1(3): 519–533
Everitt B L (1980). Ecology of saltcedar — a plea for research. Environmental Geology, 3(2): 77–84
Everitt J H, Deloach C J (1990). Remote sensing of Chinese tamarisk (Tamarix chinensis) and associated vegetation. Weed Sci, 38: 273–278
Fielding A H, Bell J F (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv, 24(1): 38–49
Finch D M, Stoleson S H (2000). Status, ecology, and conservation of the South-western Willow Flycatcher. General Technical Report RMRS-GTR-60. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station
Hatten J R, Paradzick C E (2003). A multiscaled model of South-western Willow Flycatcher breeding habitat. JWildl Manage, 67(4): 774–788
Hatten J R, Paxton E H, Sogge M K (2010). Modeling the dynamic habitat and breeding population of Southwestern Willow Flycatcher. Ecol Modell, 221(13–14): 1674–1686
Hultine K R, Belnap J, van Riper C III, Ehleringer J R, Dennison P E, Lee M E, Nagler P L, Snyder K A, Uselman S M, West J B (2009). Tamarisk biocontrol in the western United States: Ecological and societal implications. Front Ecol Environ, 8(9): 467–474
Innes J, Barker G (1999). Ecological consequences of toxin use for mammalian pest control in New Zealand: An overview. N Z J Ecol, 23: 111–127
Jiménez-Valverde A, Lobo J M(2007). Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecol, 31(3): 361–369
Kauth R J, Thomas G S (1976). The tasseled cap — a graphic description of the spectral-temporal development of agricultural crops as seen in Landsat. In: Proceedings of the Symposium on Machine Processing of Remotely Sensed Data; LARS, Purdue University: West Lafayette, IN, USA, 41–51
Kumar S, Spaulding S A, Stohlgren T J, Hermann K A, Schmidt T S, Bahls L L (2008). Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front Ecol Environ, 7(8): 415–420
Kumar S, Stohlgren T J (2009). Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. Journal of Ecology and the Natural Environment, 1: 94–98
Leica ERDAS Imagine 9.1 (2009). Leica Geosystems Geospatial Imaging, LLC: Atlanta G A, USA, 1991–2005; available online: http://www.erdas.com
Lite S J, Stromberg J C (2005). Surface water and ground-water thresholds for maintaining Populus-Salix forests, San Pedro River, Arizona. Biol Conserv, 125(2): 153–167
Liu C, Berry P M, Dawson T P, Pearson R G (2005). Selecting thresholds of occurrence in the prediction of species distributions. Ecography, 28(3): 385–393
Lytle D A, Merritt D M (2004). Hydrologic regimes and riparian forests: A structured population model for cottonwood. Ecology, 85(9): 2493–2503
Matarczyk J A, Willis A J, Vranjic J A, Ash J E (2002). Herbicides, weeds and endangered species: Management of bitou bush (Chrysanthemoides monilifera spp. rotundata) with glyphosate and impacts on the endangered shrub, Pimelea spicata. Biol Conserv, 108(2): 133–141
Merritt D M, Poff N L R (2010). Shifting dominance of riparian Populus and Tamarix along gradients of flow alteration in western North American rivers. Ecol Appl, 20(1): 135–152
Morisette J T, Jarnevich C S, Ullah A, Cai W, Pedelty J A, Gentle J E, Stohlgren T J, Schnase J L (2006). A tamarisk habitat suitability map for the continental United States. Front Ecol Environ, 4(1): 11–17
Nagler P L, Glenn E P, Huete A R (2001). Assessment of spectral vegetation indices for riparian vegetation in the Colorado River delta, Mexico. J Arid Environ, 49(1): 91–110
Naiman R J, Décamps H (1997). The ecology of interfaces: Riparian zones. Annu Rev Ecol Syst, 28(1): 621–658
Naiman R J, Décamps H, Pollock M (1993). The role of riparian corridors in maintaining regional biodiversity. Ecol Appl, 3(2): 209–212
Patten D T (1998). Riparian ecosystems of semi-arid North America: Diversity and human impacts. Wetlands, 18(4): 498–512
Paxton E H, Sogge MK, Durst S L, Theimer T C, Hatten J R (2007). The ecology of the Southwestern Willow Flycatcher in central Arizona — a 10-year synthesis report: US. Geological Survey Open-File Report 2007-1381
Pearce J, Ferrier S (2000). Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Modell, 133(3): 225–245
Phillips S J, Anderson R P, Schapire R E (2006). Maximum entropy modeling of species geographic distributions. Ecol Modell, 190(3–4): 231–259
Robinson TW(1965). Introduction, spread, and areal extent of saltcedar (Tamarix) in the western states. US Geological Survey, Washington D C, USA
Shafroth P B, Briggs M K (2008). Restoration ecology and invasive riparian plants: An introduction to the special section on Tamarix spp. in western North America. Restor Ecol, 16(1): 94–96
Sogge M K, Marshall R M (2000). A survey of current breeding habitats. In: Finch D M, Stoleson S H, eds. Status, ecology, and conservation of the South-western Willow Flycatcher. USDA Forest Service General Technical Report RMRS-GTR-60. USDA Forest Service Rocky Mountain Research Station, Ogden, UT, 43–56
Sogge M K, Marshall R M, Sferra S J, Tibbitts T J (1997). A Southwestern Willow Flycatcher natural history summary and survey protocol. USGS Biological Resources Division, Colorado Plateau Research Station, Northern Arizona University
Sogge M K, Sferra S J, Paxton E H (2008). Tamarix as habitat for birds: Implications for riparian restoration in the southwestern United States. Restor Ecol, 16(1): 146–154
Song C, Woodcock C E, Seto K C, Lenney M P, Macomber S A (2001). Classification and change detection using Landsat TM data: When and how to correct atmospheric effects? Remote Sens Environ, 75(2): 230–244
Song C H, Woodcock C E (2003). Monitoring forest succession with multitemporal Landsat images: Factors of uncertainty. IEEE Trans Geosci Rem Sens, 41(11): 2557–2567
Stohlgren T J, Bull K A, Otsuki Y, Villa C, Lee M (1998). Riparian zones as havens for exotic plant species in the central grasslands. Plant Ecol, 138(1): 113–125
Stromberg J C, Beauchamp V B, Dixon M D, Lite S J, Paradzick C (2007a). Importance of low-flow and high-flow characteristics to restoration of riparian vegetation along rivers in arid south-western United States. Freshw Biol, 52(4): 651–679
Stromberg J C, Lite S J, Marler R, Paradzick C, Shafroth P B, Shorrock D, White J M, White M S (2007). Altered stream-flow regimes and invasive plant species: the Tamarix case. Glob Ecol Biogeogr, 16(3): 381–393
Szaro R C, Rinne J N (1988). Ecosystem approach to management of southwestern riparian communities. Transactions of the 53rd North American Wildlife and Natural Resources Conference, 53: 502–511
Taylor J P, McDaniel K C (1998). Restoration of saltcedar (Tamarix sp.)-infested floodplains on the Bosque del Apache National Wildlife Refuge. Weed Technol, 12: 345–352
Unitt P (1987). Empidonax traillii extimus: an endangered subspecies. West Birds, 18: 137–162
United States Fish and Wildlife Service (1995). Final rule determining endangered status for the Southwestern Willow Flycatcher. Fed Regist, 60: 10694–10715
Vitousek P M, DAntonio C M, Loope L L, Rejmanek M, Westbrooks R. (1997). Introduced species: A significant component of humancaused global change. N Z J Ecol, 21: 1–16
Zavaleta E S (2000). The economic value of controlling an invasive shrub. AMBIO: A Journal of the Human Environment, 29: 462–467
Zavaleta E S, Hobbs R J, Mooney H A (2001). Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol, 16(8): 454–459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
York, P., Evangelista, P., Kumar, S. et al. A habitat overlap analysis derived from maxent for tamarisk and the south-western willow flycatcher. Front. Earth Sci. 5, 120–129 (2011). https://doi.org/10.1007/s11707-011-0154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11707-011-0154-5