Abstract
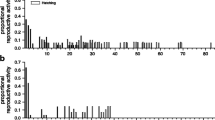
Variation in reproductive success is most pronounced in species with strongly biased operational sex ratios, prominent sexual dimorphisms, and where mate competition and choice are likely. We studied sexual selection in eastern tiger salamanders (Ambystoma t. tigrinum) and examined the role of body size on reproductive success. We genotyped 155 adults and 1,341 larvae from 90 egg masses at six microsatellite loci. Parentage analyses revealed both sexes engaged in multiple matings, but was more common among females (64%) than males (27%). However, the standardized variance in mating and reproductive success was higher in males. Bateman gradients were significant and nearly identical in both sexes, suggesting that sexual selection was roughly equal between sexes. Body size was not correlated with mating or reproductive success in either sex. The apparent lack of sexual selection on body size may be a result of sperm storage, sperm competition, alternative mating tactics, and/or random induction of spermatophores.


Similar content being viewed by others
References
Andersson, M. (1982). Female choice selects for extreme tail length in a widowbird. Nature, 299, 818–820. doi:10.1038/299818a0.
Arnold, S. J. (1976). Sexual behavior, sexual interference, and sexual defense in the salamanders Ambystoma maculatum, Ambystoma tigrinum, and Plethodon jordani. Zeitschrift fur Tierpsychologie, 42, 247–300.
Arnold, S. J., & Duvall, D. (1994). Animal mating systems—A synthesis based on selection theory. American Naturalist, 143, 317–348. doi:10.1086/285606.
Bateman, A. J. (1948). Intrasexual selection in Drosophila. Heredity, 2, 349–368. doi:10.1038/hdy.1948.21.
Boisseau, C., & Joly, J. (1975). Transport and survival of spermatozoa in female Amphibia. In E. S. E. Hafez & C. G. Thibault (Eds.), The biology of spermatozoa (pp. 94–104). Karger, Switzerland: Basel.
Bos, D. H., Williams, R. N., Gopurenko, D., Bulut, Z., & DeWoody, J. A. (2009). Condition dependent mate choice and a reproductive advantage for MHC-divergent male tiger salamanders. Molecular Ecology (in press).
Brunton, D. H., Evans, B., Cope, T., & Ji, W. (2008). A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): Female neighbors as threats. Behavioral Ecology, 19, 791–798. doi:10.1093/beheco/arn027.
Chandler, C. H., & Zamudio, K. R. (2008). Reproductive success by large, closely related males facilitated by sperm storage in an aggregate breeding amphibian. Molecular Ecology, 17, 1564–1576. doi:10.1111/j.1365-294X.2007.03614.x.
Clutton-Brock, T. H. (1988). Reproductive success. In T. H. Clutton-Brock (Ed.), Reproductive success (pp. 472–485). Chicago: University of Chicago Press.
Clutton-Brock, T. H. (2007). Sexual selection in males and females. Science, 318, 1882–1885. doi:10.1126/science.1133311.
DeWoody, J. A. (2005). Molecular approaches to the study of parentage, relatedness and fitness: Practical applications for wild animals. The Journal of Wildlife Management, 69, 1400–1418. doi:10.2193/0022-541X(2005)69[1400:MATTSO]2.0.CO;2.
Douglas, M. E., & Monroe, B. L., Jr. (1981). A comparative study of topographical orientation in Ambystoma (Amphibia: Caudata). Copeia, 198, 460–463. doi:10.2307/1444239.
Duchesne, P., Godbout, M. H., & Bernatchez, L. (2002). PAPA: A computer program for simulated and real parental allocation. Molecular Ecology Notes, 2, 191–194. doi:10.1046/j.1471-8286.2002.00164.x.
Eberhard, W. G. (1996). Female control: Sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press.
Emlen, S. T., & Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science, 197, 215–223. doi:10.1126/science.327542.
Fedorka, K. M., & Mousseau, T. A. (2002). Material and genetic benefits of female multiple mating and polyandry. Animal Behaviour, 64, 361–367. doi:10.1006/anbe.2002.3052.
Gabor, C. R., & Halliday, T. R. (1997). Sequential mate choice by smooth newts: Females become more choosy. Behavioral Ecology, 8, 162–166. doi:10.1093/beheco/8.2.162.
Gabor, C. R., Krenz, J. D., & Jaeger, R. G. (2000). Female choice, male interference, and sperm precedence in the red-spotted newt. Behavioral Ecology, 11, 115–124. doi:10.1093/beheco/11.1.115.
Gill, D. E. (1978). The metapopulation ecology of the red-spotted newt, Notophthalmus viridescens. Ecological Monographs, 48, 145–166. doi:10.2307/2937297.
Gladstone, D. E. (1979). Promiscuity in monogamous colonial birds. American Naturalist, 114, 545–557. doi:10.1086/283501.
Gopurenko, D., Williams, R. N., & DeWoody, J. A. (2007). Reproductive and mating success in the small-mouthed salamander (Ambystoma texanum) estimated via microsatellite parentage analysis. Evolutionary Biology, 34, 130–139. doi:10.1007/s11692-007-9009-0.
Gopurenko, D., Williams, R. N., McCormick, C. R., & DeWoody, J. A. (2006). Insights into the mating habits of the tiger salamander (Ambystoma tigrinum tigrinum) as revealed by genetic parentage analyses. Molecular Ecology, 5, 1917–1928. doi:10.1111/j.1365-294X.2006.02904.x.
Gowaty, P. A. (2004). Sex roles, contests for the control of reproduction, and sexual selection. In P. Kappeler & C. van Schaik (Eds.), Sexual selection in primates: New and comparative perspectives (pp. 37–54). Cambridge, UK: Cambridge University Press.
Gowaty, P. A., & Hubbell, S. P. (2005). Chance, time allocation, and the evolution of adaptively flexible sex role behavior. Integrative and Comparative Biology, 45, 931–944. doi:10.1093/icb/45.5.931.
Halliday, T. R. (1983). The study of mate choice. In P. Bateson (Ed.), Mate choice. Cambridge: Cambridge University Press.
Halliday, T. (1998). Sperm competition in amphibians. In T. R. Birkhead & A. P. Moller (Eds.), Sperm competition and sexual selection. SanDiego: Academic Press.
Halliday, T., & Arnold, S. J. (1987). Multiple mating by females: A perspective from quantitative genetics. Animal Behaviour, 35, 939–941. doi:10.1016/S0003-3472(87)80138-0.
Harris, W. E., & Lucas, J. R. (2002). A state-based model of sperm allocation in a group-breeding salamander. Behavioral Ecology, 13, 705–712. doi:10.1093/beheco/13.5.705.
Houck, L. D. (1988). The effect of body size on male courtship success in a plethodontid salamander. Animal Behaviour, 36, 837–842. doi:10.1016/S0003-3472(88)80166-0.
Houck, L. K., & Arnold, S. J. (2003). Courtship and mating behavior. In D. M. Sever (Ed.), Reproductive biology and phylogeny of Urodela (pp. 383–424). Enfield, NH: Science Publishers Inc.
Houck, L. D., Tilley, S. G., & Arnold, S. J. (1985). Sperm competition in a plethodontid salamander: Preliminary results. Journal of Herpetology, 19, 420–423. doi:10.2307/1564273.
Howard, R. D., Moorman, R. S., & Whiteman, H. H. (1997). Differential effects of mate competition and mate choice on eastern tiger salamanders. Animal Behaviour, 53, 1345–1356. doi:10.1006/anbe.1996.0359.
Hubbell, S. P., & Johnson, S. K. (1987). Environmental variance in lifetime mating success, mate choice, and sexual selection. American Naturalist, 130, 91–112. doi:10.1086/284700.
Humphrey, R. R. (1977). Factors influencing ovulation in the Mexican axolotyl as revealed by induced spawning. The Journal of Experimental Zoology, 199, 209–214. doi:10.1002/jez.1401990205.
Janzen, F. J., & Brodie, E. D., III (1989). Tall tails and sexy males: Sexual behavior of rough-skinned newts (Taricha granulosa) in a natural breeding pond. Copeia, 1989, 1068–1071. doi:10.2307/1446002.
Jones, A. G., Adams, E. M., & Arnold, S. J. (2002a). Topping off: A mechanism of first-mail sperm precedence in a vertebrate. Proceedings of the National Academy of Sciences of the United States of America, 99, 2078–2081. doi:10.1073/pnas.042510199.
Jones, A. G., Arguello, J. R., & Arnold, S. J. (2002b). Validation of Bateman’s principles: A genetic study of sexual selection and mating patterns in the rough-skinned newt. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269, 2533–2539. doi:10.1098/rspb.2002.2177.
Jones, A. G., Arguello, J. R., & Arnold, S. J. (2004). Molecular parentage analysis in experimental newt populations: The response of mating system measures to variation in the operational sex ratio. American Naturalist, 164, 444–456. doi:10.1086/423826.
Jones, B., Grossman, G. D., Walsh, D. C. I., Porter, B. A., Avise, J. C., & Fiumera, A. C. (2007). Estimating differential reproductive success from nests of related individuals, with application to a study of the mottled sculpin, Cottus bairdi. Genetics, 176, 2427–2439. doi:10.1534/genetics.106.067066.
Jones, A. G., Rosenqvist, G., Berglund, A., & Avise, J. C. (2005). The measurement of sexual selection using Bateman’s principles: An experimental test in the sex-role-reversed pipefish Syngnathus typhle. Integrative and Comparative Biology, 45, 874–884. doi:10.1093/icb/45.5.874.
Ketterson, E. D., Parker, P. G., Raouf, S. A., Nolan, V., Ziegenfus, C., & Chandler, C. R. (1998). Bateman’s paradigm as a case study. In P. G. Parker & N. T. Burley (Eds.), Avian reproductive tactics: Female and male perspectives (pp. 81–101). Lawrence, KS: Allen Press.
Kumpf, K. F. (1934). The courtship of Ambystoma tigrinum. Copeia, 1934, 7–10. doi:10.2307/1436425.
Levitan, D. R. (1998). Sperm limitation, sperm competition and sexual selection in external fertilizers. In T. Birkhead & A. Moller (Eds.), Sperm competition and sexual selection (pp. 173–215). New York: Academic Press.
Levitan, D. R. (2005). The distribution of male and female reproductive success in a broadcast spawning marine invertebrate. Integrative and Comparative Biology, 45, 848–855. doi:10.1093/icb/45.5.848.
Lorch, P. D., Bussière, L., & Gwynne, D. T. (2008). Quantifying the potential for sexual dimorphism using upper limits on Bateman gradients. Behaviour, 145, 1–24.
Marshall, T. C., Slate, J., Kruuk, L. E. B., & Pemberton, J. M. (1998). Statistical confidence for likelihood-based paternity inference in natural populations. Molecular Ecology, 7, 639–655. doi:10.1046/j.1365-294x.1998.00374.x.
Mech, S. G., Storfer, A., Ernst, J. A., Reudink, M. W., & Maloney, S. D. (2003). Polymorphic microsatellite loci for tiger salamanders, Ambystoma tigrinum. Molecular Ecology Notes, 3, 79–81. doi:10.1046/j.1471-8286.2003.00356.x.
Morrissey, M. B., & Wilson, A. J. (2005). The potential cost of accounting for genotypic errors in molecular parentage analysis. Molecular Ecology, 14, 4111–4121. doi:10.1111/j.1365-294X.2005.02708.x.
Newcomer, S. D., Zeh, J. A., & Zeh, D. W. (1999). Genetic benefits enhance the reproductive success of polyandrous females. Proceedings of the National Academy of Sciences of the United States of America, 96, 10236–10241. doi:10.1073/pnas.96.18.10236.
Olsson, M. (1993). Male preference for large females and assortative mating for body size in the sand lizard (Lacerta agilis). Behavioral Ecology and Sociobiology, 32, 337–341. doi:10.1007/BF00183789.
Parra-Olea, G., Recuero, E., & Zamudio, K. R. (2007). Polymorphic microsatellite markers for Mexican salamanders of the genus Ambystoma. Molecular Ecology Notes, 7, 818–820. doi:10.1111/j.1471-8286.2007.01714.x.
Peakall, R., & Smouse, P. E. (2006). Genalex 6: Genetic analysis in excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6, 288–295. doi:10.1111/j.1471-8286.2005.01155.x.
Peckham, R. S., & Dineen, C. F. (1954). Spring migrations of salamanders. Proceedings of the Indiana Academy of Sciences, 64, 278–280.
Petranka, J. A. (1998). Salamanders of the United States and Canada. Washington, DC: Smithsonian Institution.
Phillips, C. A., & Sexton, O. J. (1989). Orientation and sexual differences during breeding migration of the spotted salamander, Ambystoma maculatum. Copeia, 1989, 17–22. doi:10.2307/1445599.
Queller, D. C., & Goodnight, K. F. (1989). Estimating relatedness using genetic markers. Evolution, 43, 258–275.
Regosin, J. J. V., Windmiller, B. S., Homan, R. N., & Reed, J. M. (2005). Variation in terrestrial habitat use by four pool-breeding amphibian species. The Journal of Wildlife Management, 69, 1481–1493. doi:10.2193/0022-541X(2005)69[1481:VITHUB]2.0.CO;2.
Rose, F. L., & Armentrout, D. (1976). Adaptive strategies of Ambystoma tigrinum (Green) inhabiting the Llano Estacado of west Texas. Journal of Animal Ecology, 45, 713–729. doi:10.2307/3577.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning: A laboratory manual (3rd ed.). Cold Springs Harbor, NY: Cold Springs Harbor Laboratory Press.
Sarhan, A., & Kokko, H. (2007). Multiple mating in the Glanville fritillary butterfly: A case of within-generation bet-hedging? Evolution; International Journal of Organic Evolution, 61, 606–616. doi:10.1111/j.1558-5646.2007.00053.x.
Schulte-Hostedde, A. I., Millar, J. S., & Gibbs, H. L. (2004). Sexual selection and mating patters in a mammal with female-biased sexual size dimorphism. Behavioral Ecology, 15, 351–356. doi:10.1093/beheco/arh021.
Semlitsch, R. D. (1998). Biological delineation of terrestrial buffer zones for pond breeding salamanders. Conservation Biology, 12, 1113–1119. doi:10.1046/j.1523-1739.1998.97274.x.
Sever, D. M. (1995). Spermathecae of Ambystoma tigrinum (Amphibia: Caudata): Development and a role for the secretions. Journal of Herpetology, 29, 243–255. doi:10.2307/1564561.
Sever, D. M. (2002). Female sperm storage in amphibians. The Journal of Experimental Zoology, 292, 165–179. doi:10.1002/jez.1152.
Sever, D. M. (Ed.). (2003). Courtship and mating glands. In Reproductive biology and Phylogeny of Urodela. New Hampshire: Science Publishers, Inc.
Sever, D. M., & Dineen, C. F. (1978). Reproductive ecology of the tiger salamander, Ambystoma tigrinum, in northern Indiana. Proceedings of the Indiana Academy of Sciences, 87, 189–203.
Sever, D. M., Rania, L. C., & Krenz, J. D. (1996). Annual cycle of sperm storage in spermathecae of the red-spotted newt, Notophthalmus viridescens (Amphbiia:Salamandridae). Journal of Morphology, 227, 155–170. doi:10.1002/(SICI)1097-4687(199602)227:2<155::AID-JMOR3>3.0.CO;2-8.
Shillington, C., & Verrell, P. (1996). Multiple mating by females is not dependent on body size in the salamander Desmognathus ocrophaeus. Amphibia-Reptilia, 17, 33–38. doi:10.1163/156853896X00261.
Shuster, S. M., & Wade, M. J. (2003). Mating systems and strategies. Princeton, NJ: Princeton University Press.
Sinervo, B., & Zamudio, K. R. (2001). The evolution of alternative reproductive strategies: Fitness differential, heritability, and genetic correlation between the sexes. The Journal of Heredity, 92, 198–205. doi:10.1093/jhered/92.2.198.
Skelly, D. K. (2002). Experimental venue and estimation of interaction strength. Ecology, 83, 2097–2101.
Skelly, D. K. (2004). Microgeographic countergradient variation in the wood frog, Rana sylvatica. Evolution; International Journal of Organic Evolution, 58, 160–165.
Snyder, B. F., & Gowaty, P. A. (2007). A reappraisal of Bateman’s classic study of intrasexual selection. Evolution; International Journal of Organic Evolution, 61, 2457–2468. doi:10.1111/j.1558-5646.2007.00212.x.
Stearns, S. C. (1992). The evolution of life histories. Oxford, UK: Oxford University Press.
Tang-Martinez, Z., & Ryder, T. B. (2005). The problem with paradigms: Bateman’s worldview as a case study. Integrative and Comparative Biology, 45, 821–830. doi:10.1093/icb/45.5.821.
Tennessen, J. A., & Zamudio, K. R. (2003). Early male reproductive advantage, multiple paternity and sperm storage in an amphibian aggregate breeder. Molecular Ecology, 12, 1567–1576. doi:10.1046/j.1365-294X.2003.01830.x.
Trauth, S. E., Sever, D. M., & Semlitsch, R. D. (1994). Cloacal anatomy of paedomorphic female Ambystoma talpoideum (Caudata: Ambystomatidae), with comments on intermorph mating and sperm storage. Canadian Journal of Zoology, 72, 2147–2157. doi:10.1139/z94-287.
Twitty, V. C. (1966). Of scientists and salamanders. San Francisco, CA: Freeman.
Verrell, P. A. (1984). Sexual interference and sexual defense in the smooth newt, Triturus vulgaris (Amphibia:Urodela: Salamandridae). Zeitschrift fur Tierpsychologie, 66, 242–254.
Verrell, P. A. (1989a). Male mate choice for fecund females in a plethodontid salamander. Animal Behaviour, 38, 1086–1088. doi:10.1016/S0003-3472(89)80150-2.
Verrell, P. A. (1989b). The sexual strategies of natural populations of newts and salamanders. Herpetology, 45, 265–281.
Verrell, P. A. (1995). Males choose larger females as mates in the salamander Desmognathus santeelah. Ethology, 99, 162–171.
Verrell, P., Halliday, T., & Griffiths, M. (1986). The annual reproductive cycle of the smooth newt (Triturus vulgaris) in England. Journal of Zoology, 210, 101–119.
Verrell, P., & McCabe, N. (1988). Field observations of the sexual behaviour of the smooth newt, Triturus vulgaris vulgaris (Amphibia: Salamandridae). Journal of Zoology, 214, 533–545.
Waser, P. M., & DeWoody, J. A. (2006). Multiple paternity in a philopatric rodent: The interaction of competition and choice. Behavioral Ecology, 17, 971–978. doi:10.1093/beheco/arl034.
Williams, R. N., & DeWoody, J. A. (2004). Fluorescent dUTP helps characterize 10 novel tetranucleotide microsatellites from an enriched salamander (Ambystoma texanum) genomic library. Molecular Ecology Notes, 4, 17–19. doi:10.1046/j.1471-8286.2003.00559.x.
Williams, R. N., Gopurenko, D., Kemp, K. R., Williams, B., & DeWoody, J. A. (2009). Breeding chronology, sexual dimorphism, and genetic diversity of congeneric ambystomatid salamanders. Journal of Herpetology (in press).
Worden, B. D., & Parker, P. G. (2001). Polyandry in grain beetles leads to greater reproductive success: Material and genetic benefits? Behavioral Ecology, 12, 761–767. doi:10.1093/beheco/12.6.761.
Zar, J. (1999). Biostatistical analysis (4th ed.). New Jersey: Prentice-Hall.
Zeh, J. A., & Zeh, D. W. (2001). Reproductive mode and the genetic benefits of polyandry. Animal Behaviour, 61, 1051–1063. doi:10.1006/anbe.2000.1705.
Acknowledgements
We thank S. Baker, L. Sheets, S. Hecht, and numerous other technicians for help in collecting salamanders. We also thank members of the DeWoody lab for helpful comments on earlier drafts of this manuscript. Animals were collected under permits issued by the Indiana Department of Natural Resources and the Purdue University animal care and use committee. Financial support was provided by Purdue University and the National Science Foundation (DEB-0514815 awarded to J.A.D.). This is Agricultural Research Programs contribution number 2007-18264 from Purdue University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, R.N., DeWoody, J.A. Reproductive Success and Sexual Selection in Wild Eastern Tiger Salamanders (Ambystoma t. tigrinum). Evol Biol 36, 201–213 (2009). https://doi.org/10.1007/s11692-009-9058-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-009-9058-7