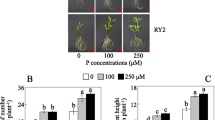
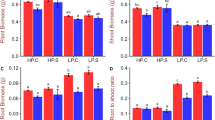
Abstract
Phosphorus is one of the essential macronutrients required for plant growth and development. The plants increase the absorption of phosphorus through an expansion of the root system in the soil when they are in low-phosphorus conditions. At the same time, the changes in biochemical metabolic pathways and the increase of secretion of phosphatase and organic acids activate the insoluble phosphate fixed in the soil. The expression profile in response to low phosphorus was investigated for rice at 6, 24 and 72 h after low-phosphorus stress compared with normal phosphorus conditions as a control with DNA chip. A total of 1207 differentially expressed genes were found in our study; 795 and 450 genes exhibited alterations in their RNA expression in response to inorganic phosphate (Pi) starvation in at least one of the three time points in roots and shoots. Thirty-eight genes overlapped in shoots and roots. The functional classification of these genes indicated their involvement in various metabolic pathways, ion transport, signal transduction, transcriptional regulation, and other processes related to growth and development. A large number of transposable elements changed the expression in rice after low-phosphorus stress. The results may provide useful information about molecular processes associated with Pi deficiency and facilitate the identification of key molecular determinants for improving Pi use by crop species.
Similar content being viewed by others
References
Ryan J, Hasan H M, Baasifi M. Availability and transformation of applied phosphorous in calcareous Lebanese soils. Soil Sci Soc Am J, 1985, 49: 1215–1220
Xiang W, Huang M, Li X. Progress on fractioning of soil phosphorous and availability of various phosphorous fractions to crops in soil. Plant Nutri Fertilizer Sci, 2004, 10: 663–670
Raghothama K G. Phosphate transport and signaling. Curr Opin Plant Biol, 2000, 3: 182–187
Vance C P, Uhde-Stone C, Allan D L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol, 2003, 157: 423–447
Zhang F, Wang J, Zhang W, et al. Nutrient use efficiencies of major cereal crops in china and measures for improvement. Acta Pedol Sin, 2008, 45: 915–924
Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta, 2002, 216: 23–27
Ticconi CA, Delatorre C A, Lahner B, et al. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J, 2004, 37: 801–814
Rubio V, Linhares F, Solano R, et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev, 2001, 15: 2122–2133
Zakhleniuk O V, Raines C A, Lloyd J C. Pho3: A phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta, 2001, 212: 529–534
Chen D, Delatorre C A, Bakker A, et al. Conditional identification of phosphate starvation-response mutants in Arabidopsis thaliana. Planta, 2000, 211: 13–22
Aung K, Lin S, Wu C, et al. Pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol, 2006, 141: 1000–1011
Bari R, Pant B D, Stitt M, et al. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol, 2006, 141: 988–999
Miura K, Rus A, Sharkhuu A, et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA, 2005, 102: 7760–7765
Duan K, Yi K, Dang L, et al. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J, 2008, 54: 965–975
Wang C, Ying S, Huang H, et al. Involvement of OsSPX in phosphate homeostasis in rice. Plant J, 2009, 57: 895–904
Maleck K, Levine A, Eulgem T, et al. The transcriptome of Arabidosis thaliana during systemic acquired resistance. Nat Genet, 2000, 26: 403–410
Kawasaki S, Borchert C, Deyholos M, et al. Gene expression profiles during the initial phase of salt stress in rice. Plant Cell, 2001, 13: 889–905
Seki M, Narusaka M, Abe H, et al. Monitoring the expression pattern of 1,300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell, 2001, 13: 61–72
Wang R, Guegler K, Labrie S T, et al. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell, 2000, 12: 1491–1509
Lian X, Wang S, Zhang J, et al. Expression profiles of 10,422 genes at early stage of low nitrogen stress in rice assayed using a cDNA microarray. Plant Mol Biol, 2006, 60: 617–631
Wasaki J, Yonetani R, Kuroda S, et al. Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant Cell Environ, 2003, 26: 1515–1523
Hammond J P, Bennett M J, Bowen H C, et al. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol, 2003, 132: 578–596
Wu P, Ma L, Hou X, et al. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol, 2003, 132: 1260–1271
Misson J, Raghothama K G, Jain A, et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA, 2005, 102: 11934–11939
Wang X, Yi K, Tao Y, et al. Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level. Plant Cell Environ, 2006, 29: 1924–1935
Yoshida S, Forno D A, Cook J H, Gomez K A. laboratory manual for physiological studies of rice. Manila: International Rice Research Institute, 1976. 61–67
Cogliatti D H, Clarkson D T. Physiological changes in, and phosphate uptake by potato plants during development of, and recovery from phosphate deficiency. Physiol Plant, 1983, 58: 287–294
Chittoor J M, Leach J E, White F F. Differential induction of peroxidase gene family during induction of rice by Xanthomonas oryzae pv. oryzae. Mol Plant-Microbe Interact, 1997, 10: 861–871
Betella M A, Quesada M A, Konowicz A K. Characterization and in situ localization of a salt-induced tomato peroxidase mRNA. Plant Mol Biol, 1994, 25: 105–114
Miller R, Zilinskas B A. Molecular cloning and characterization of a gene edcoding pea cytosolic ascorbate peroxidase. J Biol Chem, 1992, 267: 21802–21807
Roosens N H, Bemard C, Leplae R, Verbruggen N. Evidence for copper homeostasis function metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens. FEBS Lett, 2004, 5: 9–16
Mir G, Domenech J, Huguet G, et al. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J Exp Bot, 2004, 55: 2483–2493
Prasad R, Dewerqifosse P, Goffeau A, et al. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet, 1995, 27: 320–329
Martín M L, Busconi L. A rice membrane-bound calcium-dependent protein kinase is activated in response to low temperature. Plant Physiol, 2001, 125: 1442–1449
Romeis T, Piedras P, Jonathan D G J. Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell, 2001, 12: 803–816
Chico J M, Raíces M, Téllez-Ińón M T, Ulloa R M. A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol, 2002, 128: 256–270
Lopez-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol, 2002, 129: 244–256
Jiang N, Bao Z, Zhang X, et al. An active DNA transposon family in rice. Nature, 2003, 421: 163–167
Nakazaki T, Okumoto Y, Horibata A, et al. Mobilization of a transposon in rice genome. Nature, 2003, 421: 170–172
He Z, Dong H, Dong J, et al. The rice Rim2 transcript accumulates in response to Magnaporthe grisea and its predicted protein produce shares similarity with TNP2-like proteins encoded by CACTA transposons. Mol Gen Genet, 2000, 264: 2–10
Loeza-Angeles H, Sagrero-Cisneros E, Lara-Zarate L, et al. Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol Lett, 2008, 30: 1713–1719
Himanen K, Vuylsteke M, Vanneste S, et al. Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA, 2004, 101: 5146–5155
Chinnusamy V, Zhu J. Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol, 2009, 12: 133–139
Jiao Y, Deng X. A genome-wide transcriptional activity survey of rice transposable element-related genes. Genome Biol 2007, 8: R28
Hashida S, Uchiyama T, Martin C, et al. Temperature-dependent change in methylation of the Antirrhinum transposon Tam3 is controlled by the activity of its transposase. Plant Cell, 2006, 18: 104–118
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, L., Qiu, X., Li, X. et al. Transcriptomic analysis of rice responses to low phosphorus stress. Chin. Sci. Bull. 55, 251–258 (2010). https://doi.org/10.1007/s11434-010-0012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-0012-y