Abstract
In a closed landfill, we investigated the diversity and ecological characters of carabid beetles to understand the ecological importance of closed landfills that have the potential as a multi-functional habitat for improving biodiversity in urbanized areas. In addition, we studied the influence of environmental factors (vegetation structure, soil) on distribution and diversity of carabid beetles. A total of 92,495 individuals representing 15 carabid species were collected from the closed landfill. Although the species richness of carabid beetles recorded in the closed landfill was not higher than the other green spaces in the city, the closed landfill could sufficiently provides a stable habitat as a semi-natural area for carabid beetles. Soil pH, Na, and tall grass plant cover influenced carabid assemblage in the closed landfill. However, other environmental variables (e.g., K+, Na+, Mg2+, bare land cover, weedy cover, and tree cover) were not correlated with carabid species composition. It is implied that in the closed landfill, which is a highly modified engineered environment, other abiotic environmental (e.g., drainage, soil texture, leachate, and landscape context, etc.) and biotic factors (e.g., intra- and interspecific competition) may have affected carabid assemblage. Although artificial drainages are essential facilities for landfill management, they are a critical factor that affects the species inhabiting the landfill. However, carabid beetles seemed to randomly fall into the artificial drainage. For successful management of closed landfills, it is very important that minimize the intervention and that develop the ecological sensitively management method.
Similar content being viewed by others
Introduction
The closed landfill is a highly engineered environment modified with nutrient-poor, clay-rich soil. Sterile soil influences the vegetation of landfills, which are indefinitely abandoned grasslands (Rebele and Lehmann 2002; Kim and Lee 2005). Sometimes these derelict areas become a green space and serve as a novel habitat, and have the potential as a multi-functional habitat for improving biodiversity in urbanized areas (Hobbs et al. 2006; Harrison and Davis 2002). In addition, restored landfills successfully provide an attractive setting as amenity land for public enjoyment and passive recreation (Simmons 1999; Young 2000).
In South Korea, approximately 13,000 closed landfills and 360 active landfills are located close to cities (communicated by a Ministry of Environment officer, November 2011). Landfills that have been restored to green space, such as public parks and forestlands, account for approximately 15 % of total closed landfills. Most closed landfills remain abandoned grassland (35 % of total closed landfills). Government and local authorities want to restore a large number of closed landfills; however, there are some serious problems (e.g., cost benefit, public perception, visual amenities, soil conditions, and landscape design). To be successful, a site also requires a source of seed and faunal species close enough to allow natural seeding and migration to occur. This prevents sites in or near built up areas from being successful.
Some aftercare and restoration approaches have been suggested to increase the ecological diversity of landfills: non-intervention, intervention followed by natural development, and habitat creation (Simmons 1992). Before any approach is chosen, environmental risk factors must be reduced and removed to provide the opportunity for progression by spontaneous succession. Therefore, a pre-development survey that determines the potential function, importance, and environmental risk factors is an essential step in the strategy for after use (Simmons 1997). There have been few studies of pre-development surveys on the ecological functions of closed landfills, including restored landfills for the conservation of local species (e.g., birds, phytophagous insects, butterflies, and plants) (Morris 2000; Rahman et al. 2011; Weiss and Murphy 1990; Gibson 1998). Among these, carabid beetles are sufficiently abundant, taxonomically and ecologically varied, and sensitive to anthropogenic disturbance to be a reliable monitoring group, and they have been widely studied in relation to land use throughout the world. In addition, carabid assemblages on urban derelict sites change their species composition and diversity with secondary succession (Small et al. 2003; Eversham et al. 1996; Do et al. 2011). A pre-development ecological survey using carabid beetles can then be performed when restoring closed landfills and establishing management practices for improving biodiversity in the city.
We monitored the diversity and ecological characters of carabid beetles to understand the importance of landfills as a green space in urbanized areas. We also investigated the relationship of carabid assemblages with environmental variables such as soil characters and vegetation structure. In addition, we tried to identify critical environmental factors and confirm their negative effects on carabid assemblages. The results should be used to influence and inform both the site engineering design and phasing and the choices of management design and techniques.
Materials and methods
Sampling sites
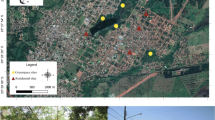
The study area, the Eulsukdo Island landfill (EIL; total area: 48.9 ha), was formerly agricultural land in the 1980s; it was then developed into a landfill in 1993, and filled with household waste. In 1997, the site reached its capacity and was covered with roughly 0.9 m of sand and clay till. We chose 10 sites in the EIL for the sampling of carabid beetles and environmental variables (e.g., soil nutrients and vegetation structures).
The EIL is surrounded with artificial drainages (length × width × height: ~10 km × 50 cm × 60 cm) for surface water treatment. They fragment the EIL into 25 landfill cells. Do et al. (2005) reported that approximately 2,000 individuals representing 25 species, including insects, soil invertebrates, earthworms, crabs, snakes, and raccoons fall into these artificial drainages and die in 1 month. Although the artificial drainages are essential facilities for landfill management, they are a critical factor that affects the species inhabiting the landfill. We randomly selected 10 sites in the artificial drainages to confirm their negative impact.
Environmental variables
Soil samples were taken at a depth of 5–10 cm from each landfill site on 21 April, 18 September, and 11 November 2011. These samples were used to establish soil chemical properties. Organic matter content (%) was determined using ash-free dry weight after ignition in a muffle furnace of 600 °C for 4 h. Soil pH was measured using a bench top probe after mixing the soil with distilled water (1:5 ratio, w/v) and filtering the extract (Whatman No. 44 filter paper). K+ (cmolc/kg), Na+ (cmolc/kg), Ca2+ (cmolc/kg), and Mg2+ (cmolc/kg) were extracted in 1 N ammonium acetate solution (pH 7.0). Exchangeable cations were measured using inductively coupled plasma–mass spectroscopy (ICP–MS, PerkinElmer, ELAN 9000 model).
The vegetation structures in three quadrants (1 m × 1 m) in the trapping area were surveyed using a Braun-Blanquet scale. Each quadrant was then assessed using four vegetation strata: bare, weedy, tall grass, and tree.
Carabid sampling
Pitfall traps were used to collect carabid beetles in the EIL. Two pitfall traps, consisting of a plastic container (length × width × height: 26 cm × 29 cm × 16 cm), partially filled with a propylene glycol–water mixture (50:50), were installed at each site. As much as possible, the traps were installed in the center of the site in homogenous stands of vegetation at each site. The trapping period covered most of the growing season (8 April–25 November 2011), and the traps were emptied once a month.
In the artificial drainages, carabid beetles were searched for in the litter and waste of 10 quadrats (50 cm × 50 cm) and all the carabids seen in a 20-min period were collected once a month. After the litter and waste were removed, the soil and sediment in the quadrats were examined in a large white tray within 5 min of the sampling to collect the carabids that might be hidden in the soil and sediment.
The ecological characters of breeding season, habitat preference, feeding type, and flight ability of each collected carabid species was derived from Do et al. (2007, 2011), Park and Paik (2001), and the Working Group for Biological Indicator Ground Beetles Database (2011). Each species was categorized according to preferred habitat (grass or forest), breeding season (spring or autumn), feeding type (herbivore or carnivore), and flight ability (flight-capable or flightless).
Data analysis
Carabid species richness of the EIL and artificial drainage were calculated using the Chao-1 estimator to estimate asymptotic species richness using 100 randomizations (without replacement) of sample accumulation order (Chao 1987). We also investigated carabid dominance structure by constructing rank-abundance plots for the EIL. Different models have been formulated to describe rank-abundance distributions, including the broken stick, geometric, log-normal, Zipf, and Zipf-Mandelbrot distribution (Kindt and Coe 2005). Fitting these distributions to the data could be reflected in an equitable state of carabid structure (Magurran 1988). These analyses were performed using BiodiversityR statistical software (Kindt and Coe 2005), which was developed for the R 2.1.1 statistical language and environment (R Development Core Team 2005).
These measurements were used in a redundancy analysis (RDA) of the relationship of measured habitat and environmental variables with the carabid assemblages (Jongman et al. 1995; ter Braak and Smilauer 2002). All environmental variables for the RDA were independently examined with a Monte-Carlo randomization test with 499 permutations. All species data were log-normalized before the RDA analyses. We centered by species when running the analysis, and performed the RDA analysis using PC-ORD (version 6, McCune and Mefford 1999).
Results
Carabid diversity and dominant structure
A total of 92,495 individuals representing 15 carabid species were collected from the EIL sites (Table 1). Nationally rare and scarce beetle species were not recorded. Total estimated species calculated by Chao-1 were 15.67 ± 0.26 species (mean ± SE) in all the EIL sites; the estimated maximum and minimum species numbers were 17 and 14.6, respectively (Fig. 1).
Dominance structure is illustrated by the rank-abundance plots constructed for the carabid beetles in the EIL. Carabid assemblages were characterized by a single dominant species and an even distribution of species (Fig. 2). Dolichus halensis, Colpodes japonicas, Amara macronota, and A. lucens made up 65 % (26,420 individuals) of the total carabid individuals at the EIL (29.8, 13.8, 11.9, and 11.7 %, respectively). Another 4 species captured (Brachinus stenoderus, Haplochlaenius costiger, and P. javanus) contributed only 2.2 % of the total carabid individuals. The fitting of various models demonstrated the carabid abundance distribution, and the log-normal curve, in particular, seemed to fit best. Akaike’s Information Criterion and Bayesian Information Criterion (AIC and BIC) statistics models that indicate the goodness-of-fit of a model for log-normal curve were also lower than the other values, which indicate better fits (cf. Fig. 2).
Species composition
Figure 3 shows that difference in carabid abundances within each ecological character (e.g., breeding season, habitat preference, feeding type, and flight ability). The autumn breeders had significantly higher abundance than summer breeders (F = 80, P < 0.001). Species numbers of autumn breeders and spring breeders did not differ, with 8 species (53.3 %) and 7 species (46.7 %), respectively. Most species (13 species, 86.7 %) preferred the grassland habitat. In addition, the abundance individuals of grassland species were significantly higher than the abundance of forest species (F = 7.13, P = 0.008). In the EIL, carnivore carabid beetles (11 species, 73.3 %) were richer than herbivore beetles (4 species, 26.7 %). However, the species abundance of carnivore species was not higher than the abundance of herbivore species (F = 0.03, P = 0.85). Although the species number of flight-capable and flightless species differed (10 flight-capable species, 66.7 % and 5 flightiness species, 33.3 %), their abundance did not differ significantly.
Relationship between carabid assemblages and environmental variables
In the RDA based on environmental variables and samples, the carabid species had eigenvalues in the first 3 axes of 0.53, 0.31, and 0.04, respectively (Fig. 4). The first 2 axes explained 88.2 % (axis 1: 55.7 % and axis 2: 32.5 %) of the variance in the relationship between carabid species and the environmental variables. The first axis of the ordination showed a separation along soil sodium content (0.6 ± 0.14 cmolc/kg mean ± standard deviation, F = 3.45, P = 0.03), while the second axis showed a gradient of soil pH (7.56 ± 0.9 mean ± standard deviation, F = 4.04, P = 0.006) and coverage with tall grass plants (68.00 ± 22.01 mean ± standard deviation, F = 3.25, P = 0.028). The other environmental variables did not significantly affect carabid assemblages (e.g., K+ 0.10 ± 0.43 cmolc/kg mean ± standard deviation; Mg2+ 1.38 ± 0.84 cmolc/kg mean ± standard deviation; coverage of trees 23.00 ± 23.12; bare land 9.0 ± 14.49).
The RDA ordination for carabids and 8 environmental variables. Carabid species are marked with lines and the environmental variables with arrows; bar coverage of bar land, TG coverage of tall grass plant, T coverage of tree plant; abbreviation of species names = ref. Table 1)
D. halensis, Lesticus magnus, and Pheropsophus javanus were relatively large-sized species associated with tall grass plant density. Herbivore species, such as A. lucens, A. macronota, Anisodactylus signatus, and A. punctatipennis, were located nearby the origin of the ordination plot. Although they were associated with various environmental variables, they did not reach statistical significance.
Effect of artificial drainage
A total of 7,223 individuals belonging to 15 species were collected from the artificial drainage of the EIL. The estimated species richness for artificial drainage was 18.45 ± 0.86 (mean ± SE); the estimated maximum and minimum species richness was 21.4 and 13.7, respectively (cf. Fig. 1a). This estimated species number is relatively higher than that of the EIL (15.67 ± 0.26). Carabid abundance in the artificial drainage accounted for 7.8 % of the total carabid beetle individuals in the EIL (cf. Fig. 1a).
The ecological characters of the carabid beetles collected in the artificial drainage could not be clearly distinguished. Many species seemed to randomly fall into the artificial drainage. More autumn breeder species were collected from the artificial drainage than spring breeders (F = 16.5, P < 0.001, cf. Fig. 3). Particularly, more flight-capable species, which may have had a greater opportunity to get away from the artificial drainage than flightless species, were collected (3,077 individuals, 42.2 % of total individuals collected from artificial drainage; F = 2.58, P = 0.11). In addition, the abundance of carnivore species, which may have been around or directly walking in the artificial drainage to find their prey, was relatively higher than herbivore species, although the difference was not significant (F = 0.18, P = 0.67).
Discussion
The species richness of carabid beetles inhabiting the EIL was not higher than the other habitats in this city (e.g., parks, fragmented forests, ravines; Do et al. 2004). Furthermore, nationally rare species were not collected. However, the EIL could sufficiently provide a stable habitat as a semi-natural area for carabid beetles. Carabid assemblage in the EIL showed a log-normal distribution. Generally, geometric distributions are found in species-poor environments, in the early stages of succession, or under highly disturbed conditions (Belaoussoff and Kevan 2003). As succession proceeds or as conditions improve, species distributions become log series and log-normal (Magurran 1988). Southwood et al. (1979) discussed that polyphagous predators (e.g., carabid beetles, rove beetles) were more influenced by the actual amount of structural heterogeneity than taxonomical plant diversity depending on successional stages of the habitat. Furthermore, although the EIL is in early succession, anthropogenic disturbances were very low because the site was abandoned and did not have a development plan for after use. By studying an abandoned paddy field that progressed through secondary succession after intense agricultural practice, Do et al. (2011) demonstrated that decreasing anthropogenic disturbance resulted in increasing carabid richness and abundance.
Many carabid beetles in the EIL preferred the grassland habitat, and many species were carnivores. In the EIL where it was not planted, the canopy was not closed. Therefore, the species richness of forest carabid species, which require the microclimatic conditions specific to forests with a closed canopy (Magura et al. 2003), was relatively lower than carabids that preferred the grassland habitat. On one hand, the increasing grass plant cover has a positive relationship with increasing the abundance of potential prey for carabid beetles. On the other hand, there were indirect effects of herb density and coverage; the high density of herbs decreased the number of species in the carabid assemblage, especially forest species. A dense coverage of grass plants may prevent the movement and food capture of the forest species, because these species are not adapted to such conditions (Sanderson et al. 1995).
Carabid assemblages were influenced by soil nutrients, especially pH and Na, in the EIL. The soil pH was such that the spatial distribution of carabids and the habitat preference was controlled by soil pH (Paje and Mossakowski 1984; Baquette 1993), especially in the egg and larvae stages, the most sensitive development stages of carabid beetles, which are very sensitive to environmental conditions (Lövei and Sunderland 1996). Additionally, the potential prey of carabids is also very sensitive to soil pH, which could further affect the abundance and species richness of carabid beetles. In this study, other environmental variables, besides pH, Na, and tall grass plant cover, were not correlated with carabid distribution and composition. It is implied that other abiotic environmental factors (e.g., drainage, soil texture, leachate, and landscape context) and biotic factors (e.g., intra- and interspecific competition) may have affected carabid assemblage (Strauss and Biedermamnn 2006; Elek et al. 2001). Among these, the landfill cover soil may be very important. In many landfills, cover soils are delivered from other derelict sites. These heterogeneous soils have a significant effect, directly and indirectly, on species inhabiting a closed landfill (Simmons 1999; Strauss and Biedermamnn 2006). Moreover, cover soil with aggregate is related to drainage and soil compaction. This soil texture influences mortality of eggs, larvae, pupae and imagos that determine the carabid diversity and distribution (Tietze 1987; Brose 2003). Therefore, the soils must also be similar to those in the surrounding areas otherwise the same species will not successfully establish.
Artificial drainage in the EIL was a critical factor that threatened the carabid assemblages. However, carabid beetles seemed to randomly fall into the artificial drainage. In 2005, when problems with the artificial drainage were reported as a threat factor to many species in the EIL, engineers and officials who managed the EIL installed many artificial corridors in the artificial drainage (Fig. 5). However, they never planned for the ecological characters and behaviors of these species. These corridors could not solve the problems. The corridors have degenerated horribly. Some ecologists and NGOs reasserted that the corridors be removed and the artificial drainage be covered using natural materials; however, officials have disregarded this (cf. Fig. 5). Only recently, a few parts of the artificial drainage were filled with aggregate. It is believed that these efforts will prevent falling into the artificial drainage, although it is not the best practice.
Conclusions
Successful management of closed landfills represents an important opportunity for increasing the ecological diversity of urbanized areas. Derelict landfill sites can harbor various carabid beetles, so for proper management of enhancing carabid diversity cover soil should be selected, intervention should be reduced for the development of tall grasslands, and proper management of drainage is needed.
References
Baquette M (1993) Habitat selection of carabid beetles in deciduous woodlands of southern Belgium. Pedobiologia 37:365–378
Belaoussoff S, Kevan PG (2003) Are there ecological foundations for ecosystem health? Environmentalist 23:255–263
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Do YH, Moon TY, Nam SH (2004) Taxonomic diversity and ecological assessment of insect fauna at River Nakdong Estuaries in Busan Metropolitan City. Nat Sci 14:107–120 (in Korean with English summary)
Do Y, Moon TY, Joo GJ (2005) Effect of artificial water channel on carabids in Eulsook Island, South Korea. In: 9th Conference of Ecology and Civil Engineering, pp 71–72
Do YN, Moon TY, Joo GJ (2007) Application of the carabid beetles as ecological indicator species for wetland characterization and monitoring in Busan and Gyeongsangnam-do. Korean J Environ Ecol 21:22–29 (in Korean with English summary)
Do Y, Jeong KS, Lineman M, Kim JY, Kim HA, Joo GJ (2011) Community changes in carabid beetles (Coleoptera: Carabidae) through ecological succession in abandoned paddy fields. J Ecol Field Biol 34:269–278
Elek Z, Magura T, Tóthmérész B (2001) Impacts of non-native Norway spruce plantation on abundance and species richness of ground beetles (Coleoptera: Carabidae). Web Ecol 2:32–37
Eversham BC, Roy DB, Telfer MG (1996) Urban, industrial and other manmade sites as analogues of natural habitats for Carabidae. Ann Zool Fennici 33:149–156
Gibson C (1998) Brownfield Red data: the values artificial habitats have for uncommon invertebrates. English Nat Res Rep 273
Harrison C, Davies G (2002) Conserving biodiversity that matters: practitioners’ perspectives on brownfield development and urban nature conservation in London. J Environ Manag 65:95–108
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecol Biogeogr 15:1–7
Jongman R, ter Braak CJF, Van Tongeren O (1995) Data analysis in community and landscape ecology. Cambridge University Press, Cambridge
Kim KD, Lee EJ (2005) Potential tree species for use in the restoration of unsanitary landfills. Environ Manag 36:1–14
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi. http://www.worldagroforestry.org/treesandmarkets/tree_diversity_analysis.asp
Lövei GL, Sunderland KD (1996) Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu Rev Entomol 41:231–256
Magura T, Tóthmérész B, Elek Z (2003) Diversity and composition of carabids during a forestry cycle. Biodiv Conserv 12:73–85
Magurran AE (1988) Ecological diversity and its measurement. Taylor & Francis, London
McCune B, Mefford MJ (1999) PC-ORD: Multivariate Analysis of Ecological Data: Version 4 for Windows. MjM Software Design
Morris MG (2000) The effects of structure and its dynamics on the ecology and conservation of arthropods in British grasslands. Biol Conserv 95:129–142
Paje F, Mossakowski D (1984) pH-preferences and habitat selection in carabid beetles. Oecologia 64:41–46
Park JK, Paik JC (2001) Coleoptera (Carabidae), Economic insects of Korea 12. National Institute of Agricultural Science and Technology, Suwon, p 169
Rahman ML, Tarrant S, McCollin D, Ollerton J (2011) The conservation value of restored landfill sites in the East Midlands, UK for supporting bird communities. Biodivers Conserv 20:1879–1893
Rebele F, Lehmann C (2002) Restoration of a landfill site in Berlin, Germany by spontaneous and directed succession. Rest Ecol 10:340–347
Sanderson RA, Rushton SP, Cherrill AJ, Byrne JP (1995) Soil vegetation and space: an analysis of their effects on the invertebrate communities of a moorland in north-east England. J Appl Ecol 32:506–518
Simmons E (1992) Landfill site restoration for wildlife. Waste Plann 3:3–7
Simmons E (1997) Improving standards of landfill restoration. Land Contam Reclam 5:63–67
Simmons E (1999) Restoration of landfill sites for ecological diversity. Waste Manage Rers 17:511–519
Small EC, Sadler JP, Telfer MG (2003) Carabid beetle assemblages on urban derelict sites in Birmingham, UK. J Insect Conserv 6:233–246
Southwood TRE, Brown VK, Reader PM (1979) The relationships of plant and insect diversities in succession. Biol J Linn Soc Lond 12:327–348
Strauss B, Biedermann R (2006) Urban brownfields as temporary habitats: driving forces for the diversity of phytophagous insects. Ecography 29:928–940
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria. http://www.R-project.org
ter Braak C, Šmilauer P (2002) CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, New York
Tietze F (1987) Changes in the structure of carabid beetle taxacenoses in grasslands affected by intensified management and industrial air-pollution. Acta Phytopathol Entomol Hung 22:305–319
Weiss SB, Murphy DD (1990) Thermal microenvironments and the restoration of rare butterfly habitat. Environmental restoration: science and strategies for restoring the earth. Island Press, Washington, DC, pp 50–60
Working Group for Biological Indicator Ground Beetles Database (2011) Natural woodlands ground beetles. Biological Indicator Ground Beetles Database, Japan. http://www.lbm.go.jp/emuseum/zukan/gomimushi/english/index.html
Young TP (2000) Restoration ecology and conservation biology. Biol Conserv 92:73–83
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government [C00168] and a 2-Year Research Grant of Pusan National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Do, Y., Kim, J.Y., Kim, GY. et al. Importance of closed landfills as green space in urbanized areas: ecological assessment using carabid beetles. Landscape Ecol Eng 10, 277–284 (2014). https://doi.org/10.1007/s11355-013-0223-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-013-0223-x